SPONSORED CONTENT
The word “innovation” gets tossed around so much these days that we almost forget its meaning—the introduction of new and different ideas for out-of-the-box problem solving that have meaningful impacts. Yet, innovation is different in every industry, and it’s essential in a field as complex as 340B.
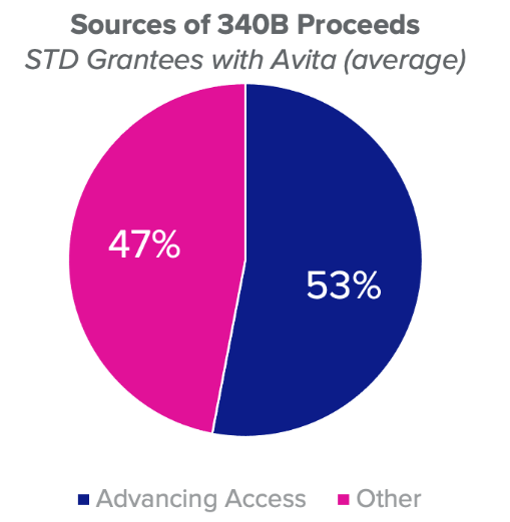
To get a handle on it, we sat down with healthcare technology veteran Andrew Stevenson, VP of Product Strategy and Business Development, and 340B industry expert Christy Bryant, VP, Customer Service & Regulatory Affairs at Macro Helix, a third-party administrator that facilitates the 340B programs of more than 900 covered entities as well as hospital-owned and retail pharmacies. Responsible for supporting customer relationships, and driving the execution of critical innovation programs with Macro Helix’s customers, they have valuable insights to share for a 340B community faced with new challenges almost daily.
In a nutshell, what is Macro Helix? How do you help customers succeed with 340B?
Christy Bryant: We help healthcare entities maximize their 340B
…