Eighty percent of all hospitals in Medicare’s outpatient prospective payment system—including 52% of all 340B hospitals—would see their net OPPS payments decline next year if Medicare ends a nearly 30% cut in 340B hospitals’ drug payments that began in 2018,
…Category: Regulatory
U.S. Public Health Service Rear Adm. Krista Pedley, a top official at the Health Resources and Services Administration (HRSA) and former longtime head of the 340B drug pricing program, has been selected by the American Society of Health-System Pharmacists (ASHP)
…Two federal health officials with long track records managing key aspects of the 340B program are playing pivotal roles in a major overhaul of the U.S. Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky last month named Mary
…U.S. Health and Human Services Secretary Xavier Becerra told members of Congress on Thursday that his department is doing all it can with the tools it has to address drug manufacturers’ denials of 340B pricing involving contract pharmacies.
If lawmakers
…The potential cost to the U.S. government next year of remedying unlawful Medicare Part B drug payment cuts for 340B hospitals could be higher than federal Medicare officials think.
The U.S. Centers for Medicare & Medicaid Services in mid-July put
…The U.S. Center for Medicare & Medicaid Services encouraged health care providers on its blog last month “to prepare for the end” of its COVID-19 waivers and flexibilities “as soon as possible and to begin moving forward to reestablishing previous
…Generic drug manufacturer Unichem Pharmaceuticals USA must repay 340B covered entities for overcharges found during a 340B program compliance audit, the U.S. Health Resources and Services Administration (HRSA) said yesterday.
HRSA has been auditing five drug manufacturers per year for
…Congress and the White House should let financially distressed critical access hospitals keep their 340B drug discounts if they opt under a new law to downgrade into emergency hospitals to remain solvent, rural health advocates told federal health officials in
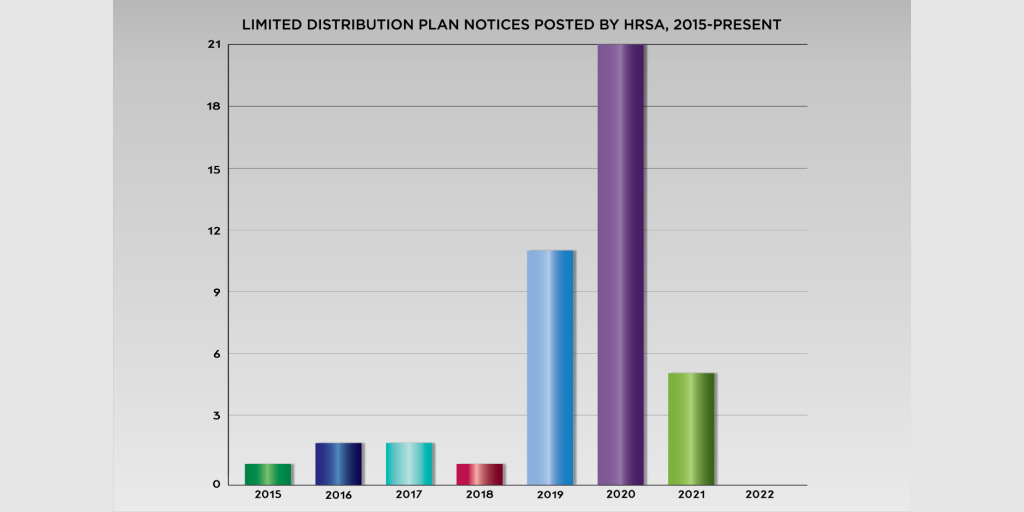
…Last September, the U.S. Health Resources and Services Administration (HRSA) published a drug manufacturers’ notice to 340B covered entities that it was limiting distribution of a medicine that alleviates a side effect of HIV/AIDS treatment.
It was the fifth limited
…