The final 340B covered entity registration period of the year began on Friday, Oct. 1 and ends on Friday, Oct. 15. Eligible hospitals, health centers, clinics, and their child sites and contract pharmacies registered during the period can begin participating
…Category: Regulatory
Breaking News
Breaking: HRSA Tells Boehringer Ingelheim its 340B Contract Pharmacy Restrictions Are Illegal
A senior federal health official informed drug manufacturer Boehringer Ingelheim (BI) today its restrictions on 340B pricing when hospitals use contract pharmacies are illegal and must end immediately, or BI could face civil monetary penalties.
U.S. Health Resources and Services
…The Biden administration this morning, as widely expected, announced it is rescinding the Trump administration’s regulatory requirement that community health centers pass along all their 340B savings on insulin and EpiPen-like devices to low-income patients with high-cost or no health
…The U.S. Health Resources and Services Administration (HRSA) says it plans to resume some onsite 340B compliance audits of covered entities starting in October. This is sparking concern among 340B providers in light of crisis conditions in some states fueled
…Hospital trade groups are lodging strong objections to maintaining steep cuts in Medicare Part B drug reimbursement as part of the Fiscal Year 2022 final rule for the hospital outpatient prospective payment system (OPPS), while the trade group for brand
…The U.S. Health Resources & Services Administration (HRSA) today referred six drug manufacturers to the U.S. Health and Human Services Department of the Office of the Inspector General (HHS OIG) regarding their refusal to offer 340B discounts to 340B covered
…The dumping of a controversial Trump administration rule limiting what 340B health centers can charge patients for insulin and EpiPen-type devices took a big step closer to being finalized last week.
Late last week Thursday, the U.S. Health Resources
…An Arkansas Insurance Department hearing officer said last week she would decide by Oct. 13 whether to stay enforcement of a state law requiring drug manufacturers to honor 340B contract pharmacy arrangements pending resolution of a bevy of federal lawsuits
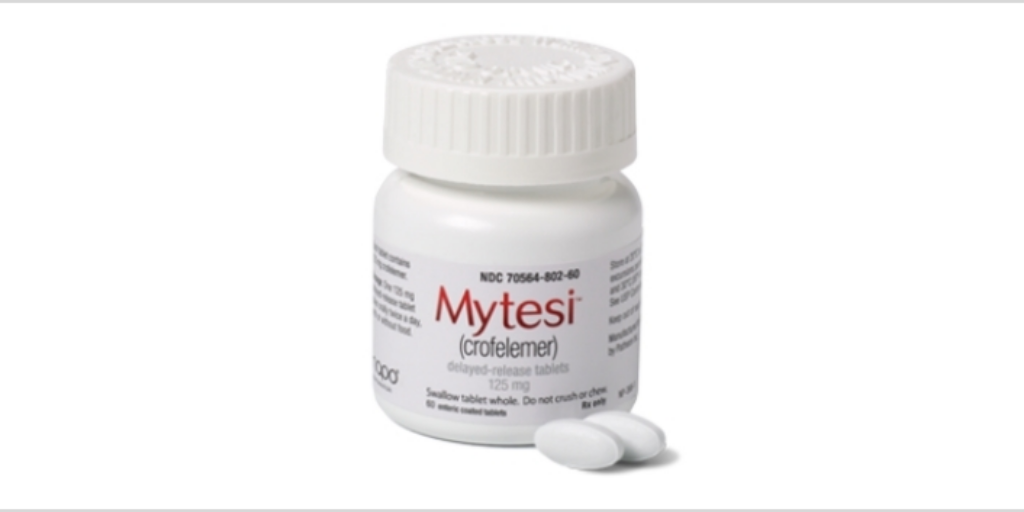
…Napo Pharmaceuticals yesterday posted a public notice to 340B covered entities about how to obtain 340B pricing on Mytesi, an antidiarrheal for patients living with HIV/AIDS who take antiretroviral drugs.
Napo said in the notice on the U.S. Health
…U.S. Health and Human Services Secretary Xavier Becerra yesterday reiterated HHS’ “strong support for the 340B Drug Pricing Program” in a 29-page plan to lower drug prices.
Becerra’s Sept. 9 report to White House domestic and economic policy counselors
…