With a two-year implementation delay nearly over, New York State is poised to fold its Medicaid managed care prescription drug benefits into its Medicaid fee for service system on April 1. State 340B health care providers that serve high volumes
…Category: Legislative
U.S. House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.) yesterday as expected announced that Rep. Brett Guthrie (R-Ky.) will chair the Subcommittee on Health and Rep. Morgan Griffith (R-Va.) the Subcommittee on Oversight and Investigations.
Guthrie said
A new U.S. House bill would put a moratorium on 340B hospital growth and impose a host of reporting requirements on 340B hospitals. It would also make it much harder for private nonprofit hospitals to remain in the 340B program.
…U.S. House Speaker Kevin McCarthy (R-Calif.) and Minority Leader Hakeem Jeffries (D-N.Y.) have reached an agreement on the balance of power in committees during this Congress and are expected to formally assign members to committees as soon as this week.
…The Medicare Payment Advisory Commission, as expected, has voted to recommend that Congress should start phasing in a new way of distributing extra Medicare payments to safety net hospitals that disproportionately serve low-income and/or uninsured beneficiaries.
The proposal that
…Oregon legislators have introduced a bill that would prohibit discriminatory practices by pharmacy benefit managers and insurance plans against 340B pharmacies, including reimbursing a 340B pharmacy less for prescription drugs compared with non-340B pharmacies.
If passed later this year, House
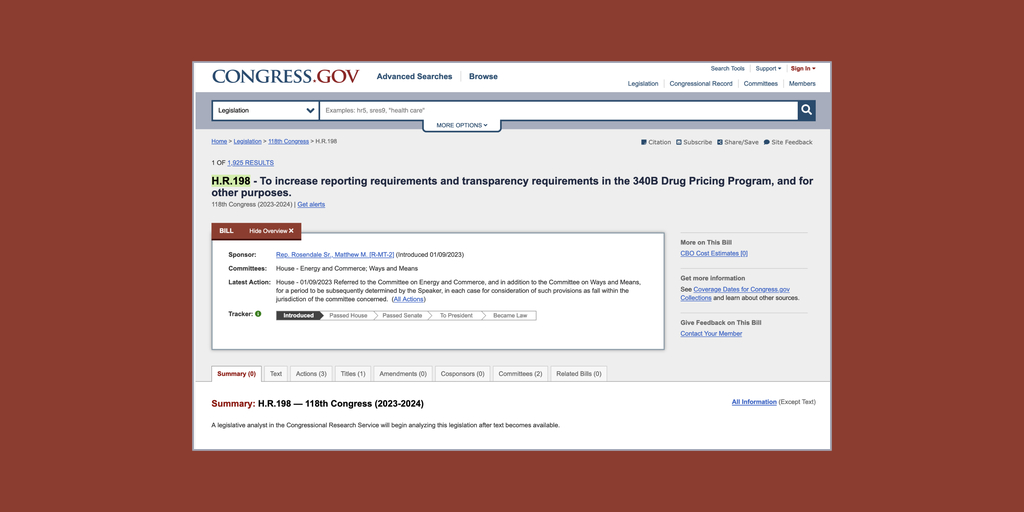
…U.S. Rep. Matt Rosendale (R-Mont.) has introduced legislation reportedly the same as he filed during the last session of Congress to impose conditions on hospital participation in the 340B program. More bills dealing with 340B are likely.
…U.S. Rep. Cathy McMorris Rodgers (R-Wash.) announced Tuesday that she was named chair of the House Energy & Commerce Committee. She in turn announced yesterday that the House Republican Steering Committee recommended nine new members to serve on the panel.
…The members, GOP chairs, and top-ranking Democrats of U.S. House committees for the 118th Congress still had not been formally announced this morning, three days after Republicans chose Rep. Kevin McCarthy (R-Calif.) to be House speaker.
It took four days
…