A note from 340B Report CEO and Publisher Ted Slafsky: Today’s issue includes the second in our ongoing series of articles by 340B Report sponsors that draw on their deep expertise. Click on the button below or reach me at ted.slafsky@340BReport.com to learn more about the benefits of becoming a sponsor.
Bipartisan Duo in Congress Wants to Protect 340B Hospitals During Pandemic
A California Democrat and Utah Republican in the U.S. House have asked their parties’ leaders to protect hospitals from losing their 340B eligibility due to the COVID-19 pandemic.
Reps. Doris Matsui (D-Calif.) and Chris Stewart’s (R-Utah) April 28 letter also asks Speaker Nancy Pelosi (D-Calif.) and Minority Leader Kevin McCarthy (D-Calif.) to support letting 340B hospitals buy covered outpatient drugs through group purchasing organizations during the pandemic.
“Our nation’s safety-net hospitals have always served on the front lines of health care for the medically underserved and uninsured,” Matsui and Stewart wrote. “During the COVID-19 pandemic, these essential providers are stepping up to provide more care and services to their communities… We ask that Congress consider providing hospitals with temporary flexibility related to 340B program eligibility rules in any future supplemental relief bill.” FierceHealthcare broke the story about their letter yesterday.
During the 2017-18 congressional session, Matsui sponsored legislation to reverse the deep cut in 340B hospitals’ Medicare Part B drug reimbursement, codify 340B’s long-standing patient definition, clarify that providers are not required to pass 340B discounts entirely to patients, and require more parity between 340B provider and drug manufacturer audits. Stewart is a member of the influential Appropriations Committee that funds federal agencies.
America’s Essential Hospitals, the American Hospital Association, and 340B Health each has asked either Congress or U.S. Health and Human Services (HHS) Secretary Alex Azar to waive the Medicare disproportionate share adjustment percentage eligibility requirement for 340B hospitals. AHA asked Azar to waive the GPO prohibition entirely while 340B Health asked the Health Resources and Services Administration (HRSA) to ease paperwork when it lets hospitals buy covered drugs through GPOs when drugs are unavailable from 340B or wholesale acquisition cost accounts. HRSA has eased reporting requirements for those GPO purchases, but still requires hospitals to keep auditable records. This is a marked shift from prior policy since the agency, along with its prime vendor Apexus, have long opposed any relaxation of the GPO prohibition rule.
Over the past decade, 340B hospital advocacy groups have been wary of bills in Congress to amend the 340B statute, even those favorable to their interests, out of fear such bills could be turned against them by drug industry supporters.
Staying Current With Clinical Pharmacy Developments Is Hard. We Have a Solution.
Due to the COVID-19 pandemic, the role of pharmacists in supporting positive patient outcomes has never been more vital. From quickly disseminating information regarding new drug therapies, to alerting providers of potential drug safety, shortages, and recalls, pharmacists act as an essential resource when making patient-specific treatment decisions.
Staying up-to-date, however, can be a challenge especially due to the number of updates and the speed information is disseminated.
RxStrategies’ Clinical Insights a complimentary resource designed to help pharmacists and other healthcare professionals stay up-to-date on the ever-changing pharmaceutical landscape and industry news. Each issue contains quick references to must-know information from leading industry sources, including:
- New Drug Approvals
- New Formulation Approvals
- New Indication Approvals
- New Drug Shortages
- New Drug Recall and Safety Alerts
- New Generic Approvals and Launches
- COVID-19 News
- Clinical and Pharmacy News
- 340B in the News
Click here for the most recent issues of RxStrategies’ Clinical Insights.
Sign-up to receive updates or visit RxStrategies.com to learn more.
White House’s Domestic Policy Leader, a Vocal Critic of 340B, Is Leaving His Post
The Wall Street Journal reported last night that White House Domestic Policy Council Director Joe Grogan is leaving his post on May 24. Grogan, the former head lobbyist for drug manufacturer Gilead, was one of the main architects of the Trump administration’s prescription drug policies.
Gilead is the maker of remdesivir, the experimental drug that shows some promise in treating COVID-19, according to a clinical trial sponsored by the National Institute of Allergy and Infectious Diseases. Also, Gilead has drawn fire for the prices of its hepatitis C treatments and HIV pre-exposure prophylaxis drugs.
In March 2019, Grogan told a meeting of the Federation of American Hospitals, the trade group for for-profit hospitals, that the 340B program sucked too much money out of patients’ and taxpayers’ pockets without fair value, and that this needed to be stopped, Inside Health Policy reported. During a Friends of Cancer Research meeting in November 2017, he said 340B was “incredibly flawed in how it was operating” and has grown “far beyond its intent” reported Politico Pro.
“Nobody who comes to CMS or the secretary’s office or talks to HRSA or comes to OMB says, ‘340B is working great and it should just continue to operate as its working and it does not need reform,’ unless they are totally divorced from reality,” he said.
That same month, Grogan said the administration was continuing deep Medicare Part B drug reimbursement cuts to 340B hospitals despite a judge’s ruling that the cuts were contrary to federal law “because the policy’s solid and we think we can win” on appeal, according to Inside Health Policy.
“We’re not going to apologize for it,” he said. “And if there’s an instance where…we’re not going to be able to get something done, at the very least it highlights a legal change that should be made and we can go to Congress and say, ‘Hey listen the courts are sticking it to us here or there and you need to clarify the law because we’re on the right track here.’”
Despite Conventional Wisdom, Few States Report Issues with 340B Duplicate Discounts, New Kaiser Study Finds
The Kaiser Family Foundation yesterday released a comprehensive state survey about how states are administering the Medicaid pharmacy benefit. It includes findings on state strategies to avoid 340B duplicate discounts and reimburse 340B-purchased drugs. Utah was the only state that did not participate.
Duplicate Discount Findings
“Despite the reported challenges of the 340B program, only five states (California, Michigan, Mississippi, New Mexico, and Rhode Island) reported issues with duplicate discounts on this survey,” Kaiser said.
The survey includes a table showing which states, as of July 1, 2019, let providers carve 340B-purchased drugs into Medicaid fee for service (FFS) and Medicaid managed care (MCO), and which ones let providers use 340B contract pharmacy in Medicaid FFS and MCO. Kaiser said only two states (New Hampshire and South Dakota) reported carving out 340B providers entirely from Medicaid FFS and only five reported carving them out entirely from Medicaid MCO (Louisiana, Nebraska, New Hampshire, North Dakota, and Washington).
The survey found that 36 states prohibit use of 340B contract pharmacy in Medicaid FFS and 19 prohibit its use in Medicaid MCO. Thirty-one states reported using National Council for Prescription Drug Programs claims indicators to flag 340B claims when they are submitted to Medicaid for payment. Seven states reported using medical claims modifiers to identify 340B claims.
The survey found that 38 states use HRSA’s Medicaid exclusion file to exclude claims from 340B providers that carve Medicaid in. The survey does not say how many and which states inappropriately use HRSA’s file to exclude 340B drugs from Medicaid MCO claims. In 2014, HRSA issued a 340B program policy release to clarify how the file should be used to prevent duplicate discounts. It said its policy applied to Medicaid FFS only, that it was working with CMS to develop a policy regarding preventing duplicate discounts in Medicaid MCO, and that states and providers should work together to develop strategies to prevent duplicate discounts on 340B drugs reimbursed through MCOs.
In January, CMS issued policy guidance on avoiding 340B duplicate discounts that observed that states could use the Medicaid state plan amendment process “to limit the ability of some or all of the covered entities and/or contract pharmacies in the state to use 340B purchased drugs for Medicaid beneficiaries. If the covered entity or contract pharmacy is not able to use 340B drugs for Medicaid beneficiaries, the pharmacy can remain a Medicaid provider and drugs can be purchased outside of the 340B program and dispensed to Medicaid patients.”
Also in January, the Government Accountability Office recommended that CMS ensure that state Medicaid programs have written policies and procedures to prevent duplicate discounts and forgone rebates. It also said HRSA should incorporate 340B covered entities’ compliance with state policies into its audits, and require covered entities to work with manufacturers regarding repayment of identified duplicate discounts in managed care.
340B Drug Reimbursement Findings
According to the survey report, “most states do not have specific requirements for 340B dispensing fees or MCO payment levels for 340B claims.” It said 42 of the 49 states that responded to the survey “indicated they did not have a dispensing fee for 340B programs that was different from the FFS professional dispensing fee, while seven did (Arizona, Illinois, Maryland, North Carolina, Oregon, South Carolina, and Tennessee).” Three states (Arizona, Iowa, and Mississippi) mandate that MCOs reimburse 340B claims at the FFS rate, it said.
Hospital Pharmacy Directors on CMS’s 340B Drug Cost Survey: Give Us a Break
As previously reported, the Centers for Medicare & Medicaid Services (CMS), despite the COVID-19 pandemic, has given hospitals until May 15 to complete a detailed survey about their 340B drug acquisition costs during the end of 2018 and beginning of 2019. That’s just 16 days from today.
CMS has said it might use the results as the basis for future Medicare Part B drug reimbursement for 340B purchased drugs. CMS is giving hospitals the option of letting CMS use 340B ceiling prices obtained from the Health Resources and Services Administration as a stand-in for average net prices hospitals actually paid. One law firm says this option might not be suitable for some hospitals because those that select it must affirm to CMS that they acquire all 340B drugs at the 340B ceiling price.
We reached out to hospital pharmacy administrators directly affected by the survey requirement for their reactions. Here are four responses:
“This is the worst time for CMS to request a response to the 340B pricing survey. We are right in the middle of the COVID-19 pandemic, with many hospitals having reduced staff. This is not the time to be attending to a very complicated survey that is estimated to take 48 hours plus (most likely double that) to formulate and submit. Also, releasing it on a Friday and having basically three weeks to complete it is not reasonable either.”
“Completing a drug acquisition survey for CMS during an unprecedented public health emergency is burdensome for hospitals that need to be razor focused on saving lives during the COVID-19 public health emergency. It has become increasingly evident that COVID-19 impacts more minorities in underserved populations. These are precisely the hospitals that would be negatively impacted from declining CMS reimbursement.”
“All of our pharmacy and 340B teams are focused on pandemic response, as well as maintaining compliance with HRSA guidance in light of rapid changes in the delivery of health care in our region. CMS is asking us to participate in a complex, time-consuming effort to cut our reimbursement rates at the very moment the continued existence of many safety net hospitals across the United States is severely threatened. The timing could not be worse or more oblivious to the condition of our health care system.”
“With COVID, everything is more difficult and a burden. It’s not business as usual in the hospitals as staff has been laid off or furloughed due to losses.”
CARES Act Uninsured Program Won’t Pay for Patients’ Outpatient Drugs
The U.S. Health and Human Services Department (HHS) has opened the online portal providers will use to file claims for CARES Act reimbursement for testing and treating uninsured patients diagnosed with COVID-19. United Health Group is administering the program under a contract with HRSA, the HHS division that also runs the 340B program.
Outpatient prescription drugs are excluded from reimbursement under the HRSA COVID-19 Uninsured Program. Their exclusion arguably elevates the importance of maximizing providers’ access to 340B drug discounts during the pandemic.
In a related development, HHS announced on April 23 that it awarded nearly $5 million through HRSA to poison control centers “to improve their capacity to respond to increased calls due to the COVID-19 pandemic.” HHS made the announcement on the same day that President Trump made headlines for pondering during a White House press briefing whether disinfectants could be injected into COVID-19 patients to knock out or clean the virus in the body. State and municipal officials reported increases in calls to poison control centers following the president’s remarks.
Tweets of Note

#340B drug acquisition cost survey has been released by @CMSGov. Read more about who must respond, what the data could be used for, instructions on how to complete the survey, and why we submitted comments urging the agency to withdraw the survey. bit.ly/3f3jvU5

NEW: Our 50 state survey on #Medicaid pharmacy benefits provides an in-depth look at how the program is administered, how states are controlling costs, supplemental rebates, 340B issues & long term priorities for states @KFF kff.org/medicaid/repor… 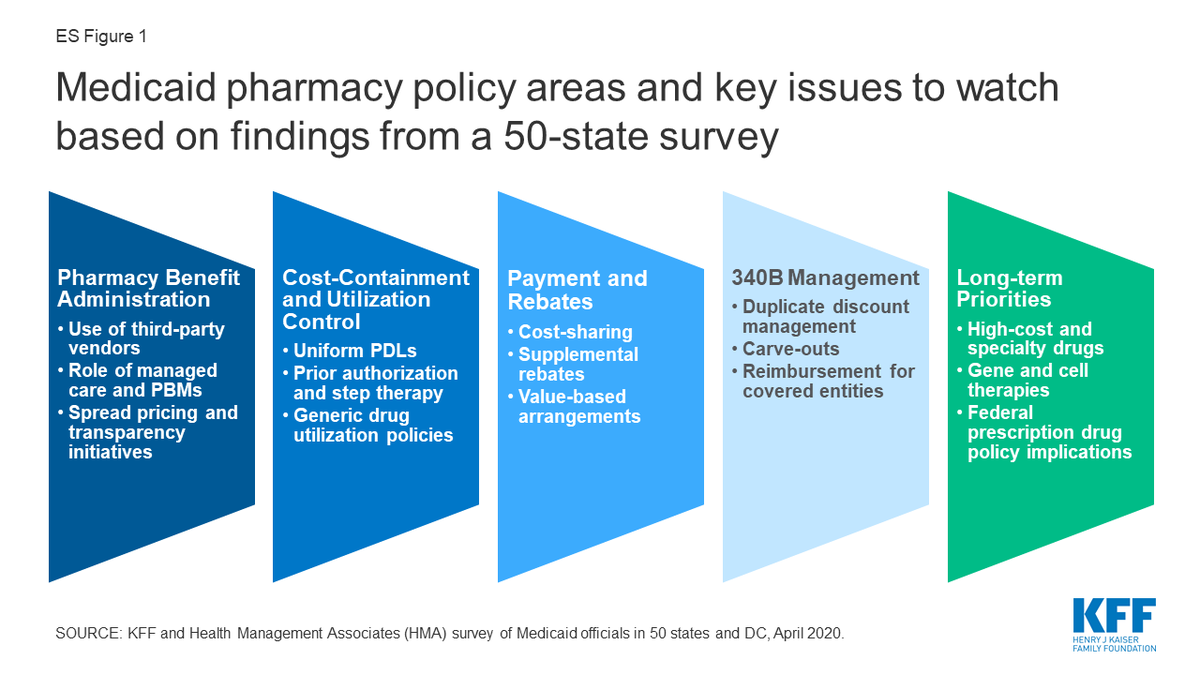

House lawmakers want new flexibility to ensure #hospitals don’t lose #340B eligibility due to #COVID19 response 

#COVID19 is only the latest indication of #healthdisparities in the U.S. #340B hospitals focus their core missions on reaching and serving marginalized communities by providing access to services tailored to their needs. bit.ly/2Kwnz0V #NationalMinorityHealthMonth

During public health crises like the #COVID19 pandemic, safety-net programs continue to support vulnerable or uninsured communities. Learn about the #340B program and how good actors like safety-net clinics put #PatientsFirst: 



