A growing number of hospitals that do not qualify for 340B drug discounts as disproportionate share hospitals are enrolling instead under less demanding criteria for rural referral centers—including nationally prominent urban hospitals in metro Boston, Chicago, and Cleveland, The Wall
…Category: Research/Reports
Thirty-five percent of drugs paid for by Medicare Part B in 2021 were administered in 340B hospital outpatient departments, up from 19% in 2012, new research funded by brand drug manufacturers shows.
The share of Part B drugs administered in
…Retail prescription drug spending in the U.S. shot up 7.8% to $378 billion in 2021, more than double the 3.7% spending growth rate in 2020, as the easing of the COVID-19 pandemic brought a surge in doctor visits and a
…Lack of data makes it hard to assess the 340B program’s overall impact on drug prices charged by pharmaceutical companies and covered entities, a new study by healthcare consulting and lobbying firm Leavitt Partners concludes.
“We found compelling evidence that
…340B hospitals use biosimilar drugs less than other hospitals, which may expose patients to higher out-of-pocket costs, a drug industry funded study concludes.
Pharmaceutical Research and Manufacturers of America released the study last week. Healthcare consulting firm Milliman did the research for
…Drug company actions to limit 340B pricing through contract and specialty pharmacies are harming 340B hospitals and their patients, according to a new American Hospital Association member survey.
AHA released the results yesterday. More than 300 hospitals were surveyed in April,
…The 340B statute should be amended to extend discounts to emergency-use drugs to treat COVID-19 as a first step in rebuilding the COVID-19 safety net, an opinion piece published yesterday in the medical journal Health Affairs says.
…Pressure on Congress to clarify the 340B program is reaching unprecedented levels, health care attorneys at the Manatt law firm said during a webinar Wednesday on the future of the program.
Meanwhile, a new survey by the firm showed that
…Congress’ nonpartisan research office has issued a four-page backgrounder on litigation over the 340B contract pharmacy program and a two-pager on the entire drug discount program. They could be a sign that 340B hearings and/or bills are coming.
The Congressional
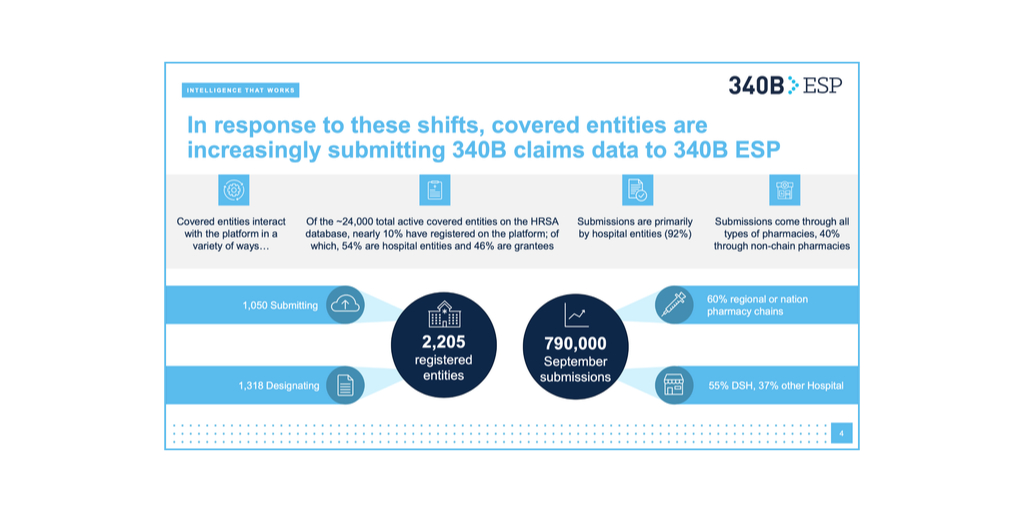
…More than 1,000 covered entities—double the number in May—are submitting 340B drug claims data to Second Sight Solutions’ 340B ESP platform to be able to keep dispensing manufacturers’ drugs to patients at multiple contract pharmacies, a senior 340B ESP leader
…