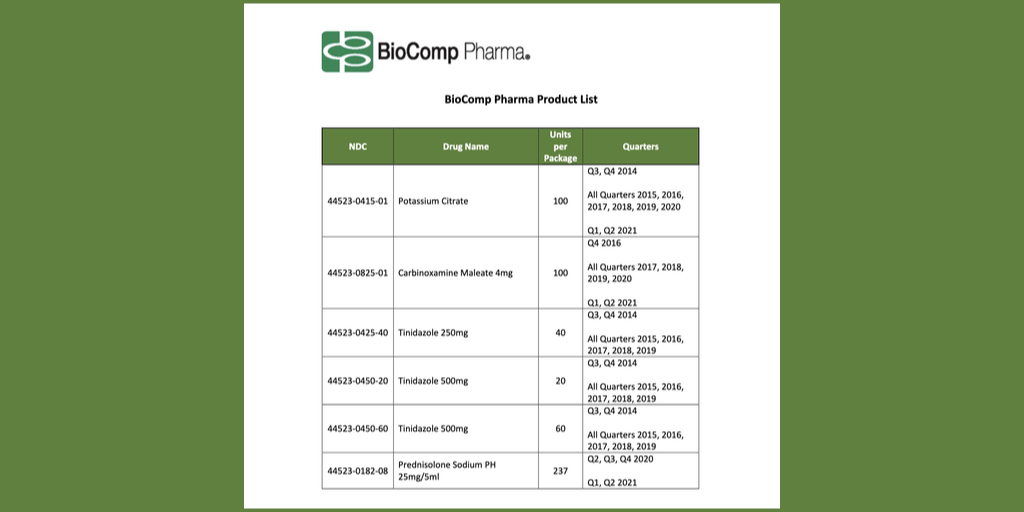
Drug manufacturer Johnson & Johnson’s new conditions on 340B-purchased drugs when hospitals use contract pharmacies apparently apply also to hospitals’ non-340B-purchased “own use” drugs, the group Ryan White Clinics for 340B Access has told its members.
J&J has not responded
…