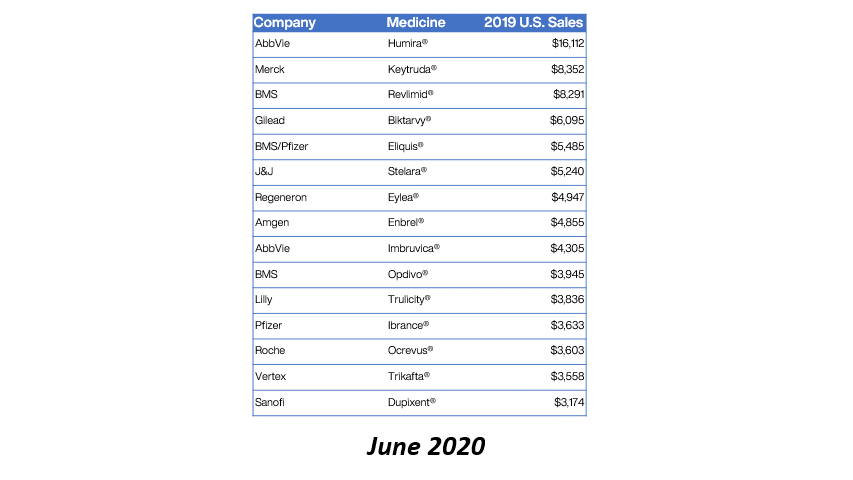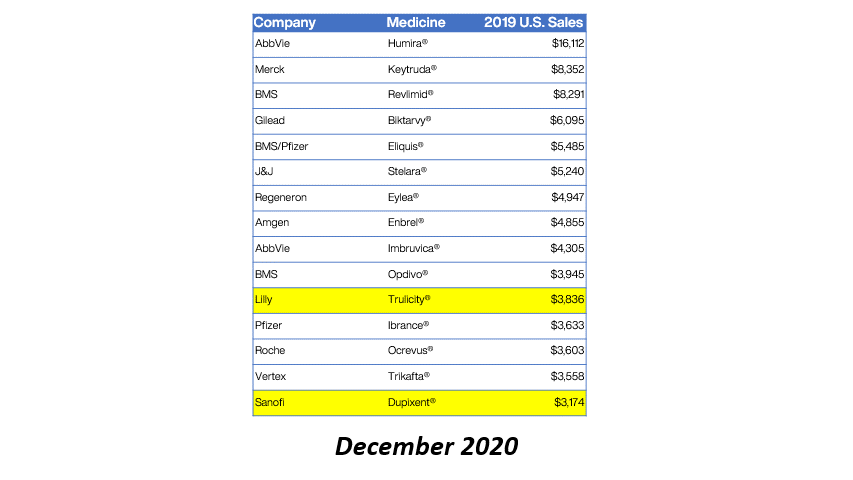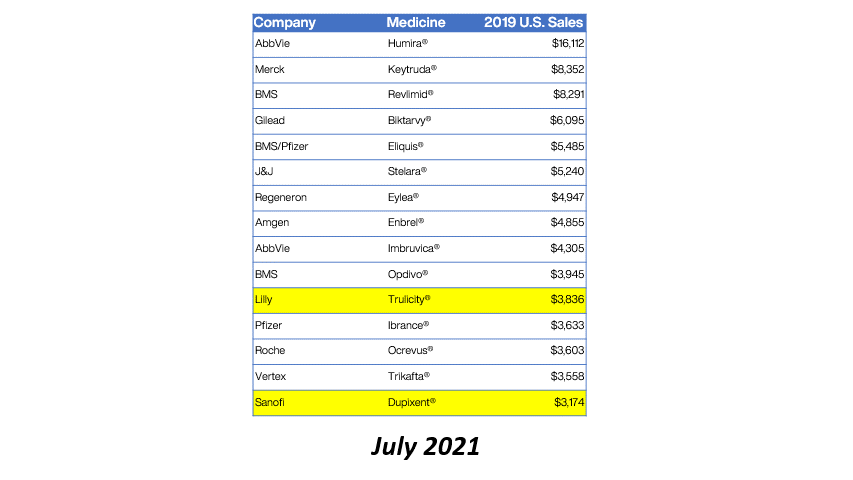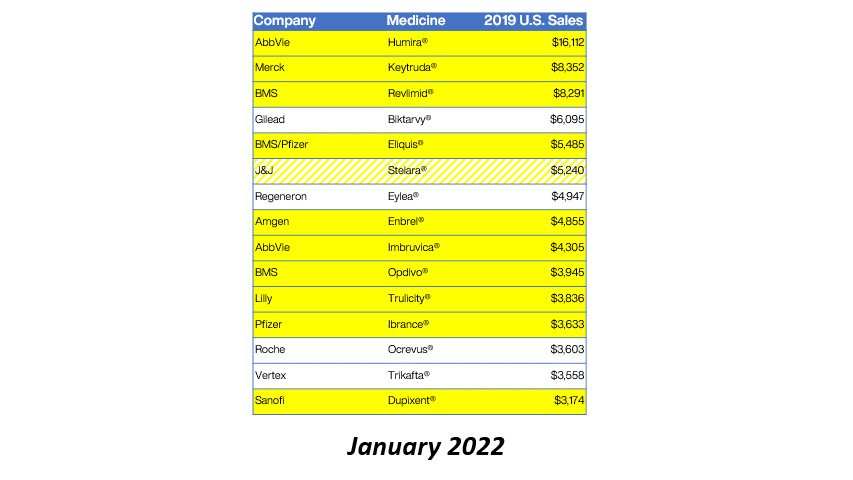
Eleven of the 15 top-selling brand drugs in the United States are now subject to manufacturer-imposed 340B pricing restrictions, including eight in the past 90 days, a new analysis shows.
Real Chemistry, a health sector communications consulting firm, published the findings in its Feb. 1 private daily email to clients. Brian Reid, leader of the firm’s corporate pricing and public affairs practice, tweeted a chart showing the figures. We are re-publishing the data with his permission.
Here’s what Reid found:
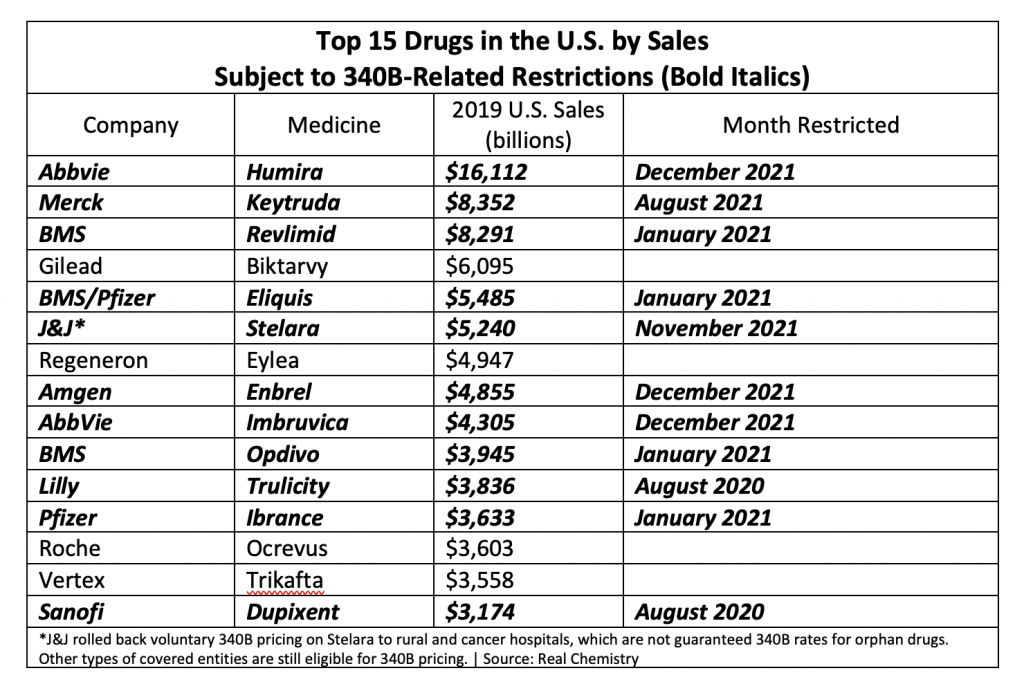
Reid said the 2019 U.S. sales figures came from the Institute for Clinical and Economic Review (ICER).
Reid’s chart includes Bristol Myers Squibb’s drug Revlimid among those subject to 340B-related restrictions. As we reported in another story today, critical access hospitals, rural referral centers, sole community hospitals, and free-standing cancer hospitals will not get voluntary 340B pricing from BMS on Revlimid, which has an orphan-drug designation and thus is subject to the 340B orphan drug exclusion. Other 340B hospitals and grantee entities will get access to 340B pricing on Revlimid, but only through one contract pharmacy that they must pick from among the specialty pharmacies in BMS’s limited distribution network for Revlimid.
Reid excluded Gilead’s HIV treatment Biktarvy from the group of 11 drugs subject to 340B pricing restrictions. Gilead has not imposed conditions on 340B pricing when covered entities use contract pharmacies, as have other companies on his list. Starting Jan. 1, however, Gilead reduced reimbursement for its HIV and hepatitis B medicines that uninsured individuals get for free through Gilead’s Advancing Access patient assistance program (PAP) to acquisition cost plus an $80 administrative fee and a $2.75 dispensing fee, compared with current reimbursement at full retail price.
Revenues from billing Gilead’s PAP for 340B drugs at above acquisition cost were a major source of funding for HIV/AIDS clinics. Gilead said the changes were needed to ensure the PAP’s sustainability.