HIV/AIDS clinics and hospitals that participate in the 340B program are condemning drug manufacturer Gilead’s new conditions on 340B pricing on its branded hepatitis C treatments when covered entities use contract pharmacies.
Ryan White Clinics for 340B Access (RWC-340B) said today it “is gravely disappointed by Gilead’s recent actions to undermine Ryan White Clinics and other safety net providers’ use of the 340B program for the communities we serve.”
“Gilead joins 14 other drug companies in putting their profits before patients by unilaterally making federal policy decisions, thumbing their noses at federal agencies, Congress, and the safety net,” said RWC-340B President Shannon Stephenson said.
Gilead’s new policy restricts 340B discounts “in a way that both the Trump and Biden administrations have called unlawful,” hospital group 340B Health President and CEO Maureen Testoni said yesterday. “Two federal courts have ruled these types of restrictions are unlawful. This behavior must stop.”
Gilead sent covered entities a letter by email late on Tuesday stating that starting May 2, 340B entities will have to provide patient claims level data for Gilead’s branded hepatitis C products to be able to continue to be eligible for bill to / ship to orders for multiple contract pharmacies. “Covered entities that decide not to participate in this integrity program, and do not have an in-house pharmacy, will have the option to select a single contract pharmacy location,” the letter says. The policy does not apply to contract pharmacies that are wholly owned by a covered entity or health system.
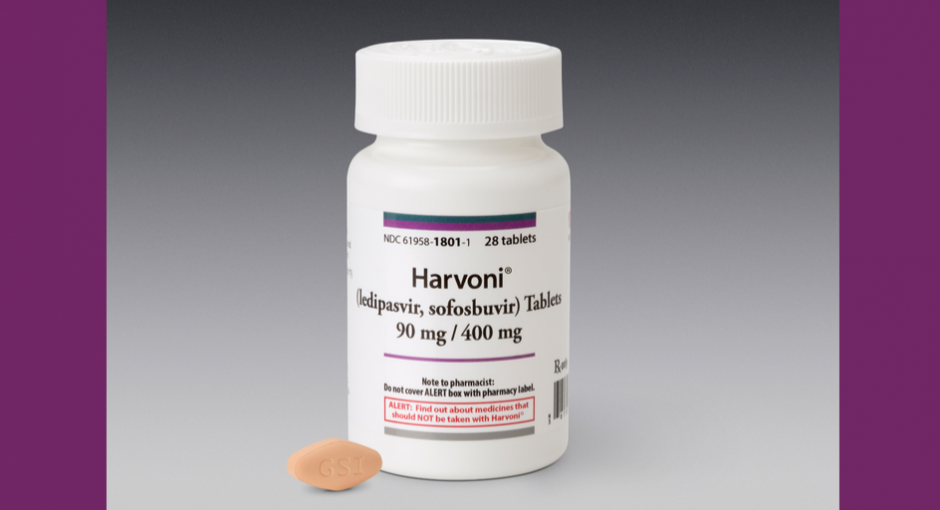
The affected products are Epclusa, Harvoni, Sovaldi, and Vosevi. Authorized generics of Epclusa (sofosbuvir / velpatasvir) and Harvoni (ledipasvir / sofosbuvir) sold by Gilead subsidiary Asegua Therapeutics are excluded.
“We are making this change across our branded hepatitis C products because of the acute impact of duplicate discounts and diversion,” the letter said.
Gilead’s new policy applies to grantee covered entities. Several manufacturers with similar 340B contract pharmacy policies have exempted non-hospital covered entities.