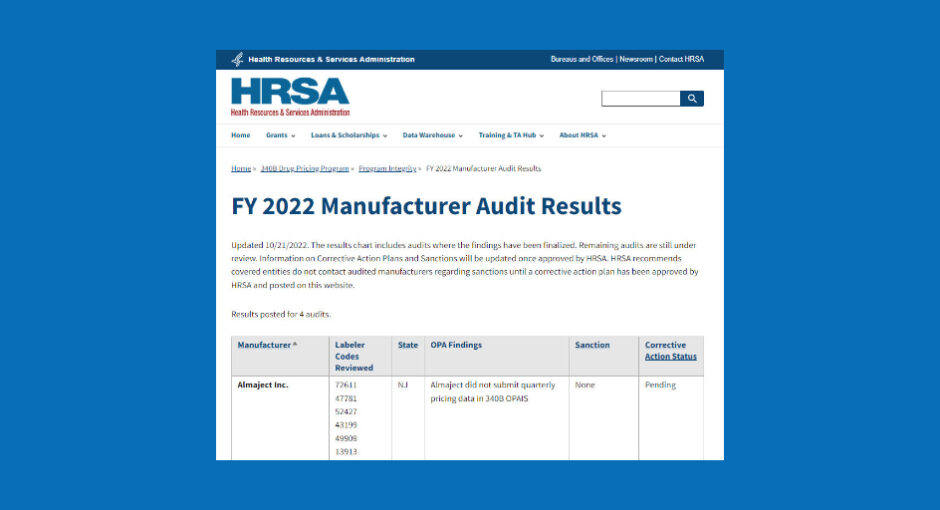
The U.S. Health Resources and Services Administration (HRSA) has cited Almaject, a New Jersey-based maker of generic injectables, with failing to submit quarterly pricing data into the 340B Office of Pharmacy Affairs Information System, although the agency did not impose sanctions on the company for the violation.
Corrective action by Almaject is still pending, according to an audit result posted on HRSA’s website October 21. The audit covered six labeler codes.
In an emailed comment, an Almaject spokesman told 340B Report, “The HRSA audit resulted in one finding … All pricing was submitted to the portal as soon as it was identified in the audit. There is nothing outstanding.” He added that the company had no information regarding HRSA’s decision to forgo sanctions.
Almaject makes generic equivalents of brand-name injectables including Clindamycin, a generic version of Pfizer’s anti-infective Cleocin Phosphate; Caspofungin Acetate, a generic equivalent of Merck’s antifungal drug Cancidas; and Dexrazoxane, a generic version of Pfizer’s detoxifying agent, Zinecard.
Almaject, based in Morristown, New Jersey, is a subsidiary of privately owned generic and OTC drug maker Alvogen. HRSA has now published results for four of the five drug manufacturers it audited during fiscal year 2022 which ended October 1.