The New Mexico House this weekend narrowly passed a bill to bar drugmakers from placing 340B contract pharmacy restrictions on [...] …
Category: Pharma Industry
Oklahoma lawmakers voted to advance a contract pharmacy access bill despite a mysterious group’s anti-340B ads, New Mexico legislators narrowed [...] …
Two pharmaceutical manufacturers have each recently added another drug to their existing 340B contract pharmacy restrictions for hospitals. Pfizer added [...] …
South Dakota today became the first state in 2025 to enact a law prohibiting drugmakers from restricting 340B contract pharmacy [...] …
Although misinformation accounts for just 2% of the overall 340B conversations taking place online, “conspiratorial narratives” driven by conservative influencers—and [...] …
As state legislatures ramp up efforts to protect contract pharmacy access, drug industry lawsuits against existing state laws continue to [...] …
A federal judge has ruled that a major hospital advocacy group and two of its members can join the government [...] …
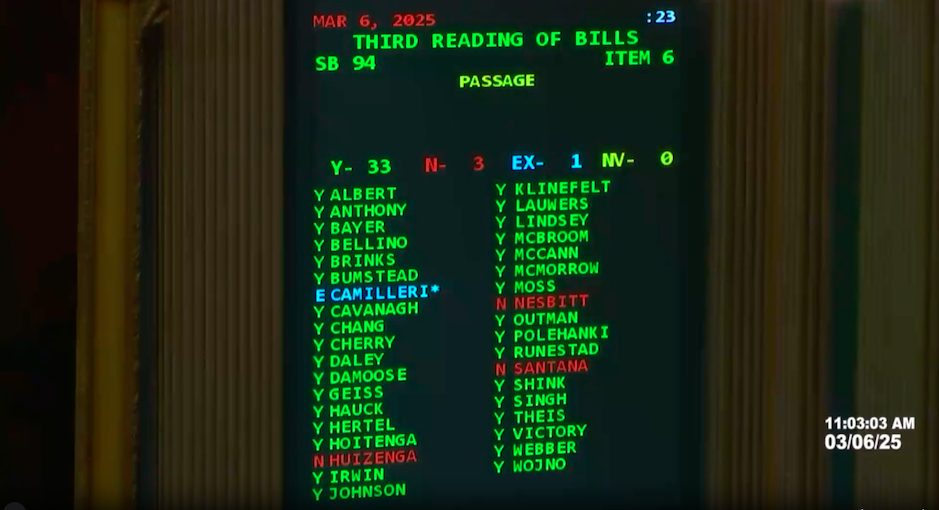
The Michigan Senate overwhelmingly approved a bill earlier today to prohibit drugmaker 340B contract pharmacy restrictions and place new reporting [...] …
The Utah House and South Dakota House both overwhelmingly voted yesterday to pass 340B contract pharmacy access legislation, sending those [...] …
A U.K.-based drug giant this week moved to add three specialty cancer treatment medications to its list of drugs subject [...] …