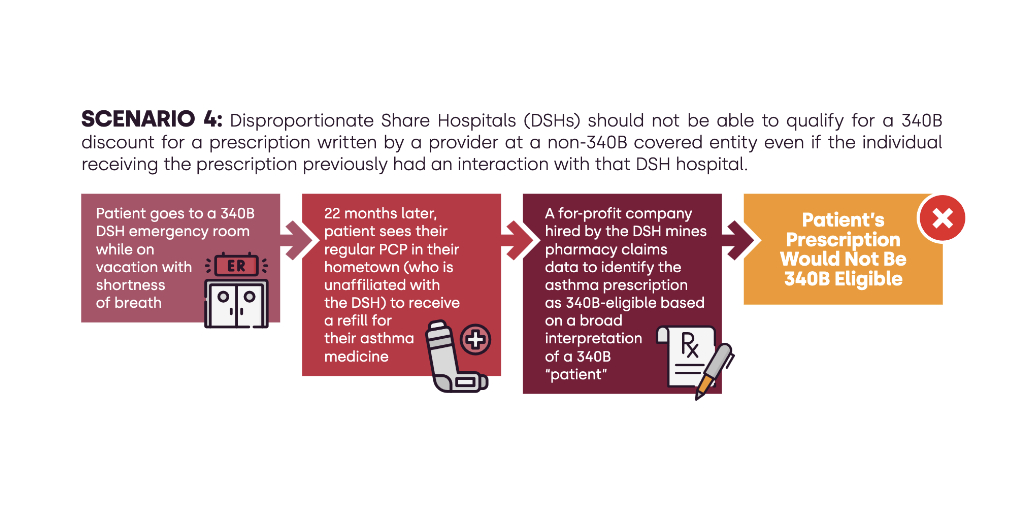
ASAP 340B, the group that drug manufacturers and community health centers formed to advance a joint 340B reform agenda, recently released a two-page explainer on how their plan would change the 340B patient definition. Under it, fewer drugs prescribed at
…Category: Pharma Industry
Drug manufacturer Merck is paying refunds for overcharges on 340B drugs purchased during the first two quarters of 2020.
Merck recently asked the U.S. Health Resources and Services Administration to post notices about its 340B ceiling price recalculations on the
…Drug manufacturer Organon, the Merck spinoff with a focus on women’s health, today became the 22nd drug maker to impose conditions on 340B pricing involving delivery to contract pharmacies.
Covered entities began receiving an email from Organon around 3:00 p.m.
…Drug manufacturer Novo Nordisk today announced that effective July 1 it will stop letting hospitals place bill to / ship to orders of 340B-purchased drugs to an unlimited number of contract pharmacies in exchange for related claims data.
It will,
…Health centers and other federal grantees’ 340B savings and how they use them need more scrutiny, as these covered entities account for a large chunk of total 340B drug sales and their margins are keeping pace with those of hospitals,
…Drug manufacturers Amgen and GlaxoSmithKline have notified 340B covered entities about refunds for overcharges, Amgen for sales in 2020 and GSK for sales in 2021.
The U.S. Health Resources and Services Administration posted both companies’ notices on its website yesterday.
…Drug manufacturer Sanofi late yesterday afternoon toughened its policy on 340B pricing involving the use of contract pharmacies. Its announcement came about two hours after Merck made a similar move.
Sanofi’s original policy applied to disproportionate share hospitals, rural
…News Alert
Merck Toughens Conditions on 340B Pricing for Hospitals and Health Centers Effective June 12
Drug manufacturer Merck today stiffened its conditions on 340B pricing when hospitals and community health centers use contract pharmacies to dispense most Merck drugs to patients.
Merck told covered entities about the changes in a letter delivered by email
…Drug manufacturer Janssen Pharmaceuticals said last week it would pay refunds for overcharges on 340B drugs purchased in the second quarter of 2020, including top-selling psoriasis drug Stelara.
Janssen, part of Johnson & Johnson, said it owes or may owe
…Generic drug manufacturers Lifestar Pharma and Chartwell Pharmaceuticals may owe refunds to 340B covered entities for overcharges on drugs purchased from the companies in the fourth quarter of 2022, according to recent notices posted on the U.S. Health Resources and
…