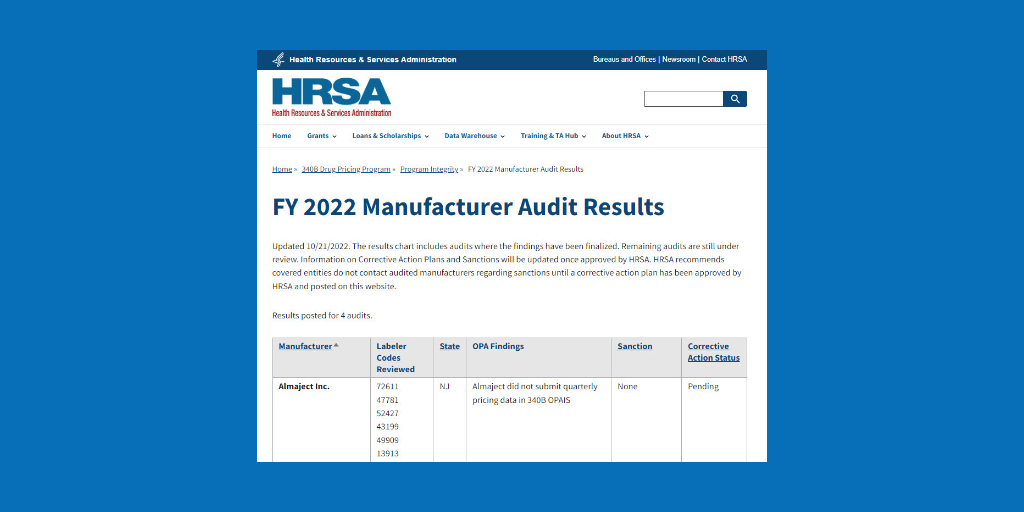
Drug manufacturers GlaxoSmithKline and Purdue Pharma have announced refunds for overcharging 340B covered entities, GSK for the fourth time and Purdue for the second time this year.
The U.S. Health Resources and Services Administration (HRSA) recently posted the companies’ notices
…