Vermont and Oregon yesterday became the latest states to enact 340B contract pharmacy access laws, with Vermont’s new statute also [...] …
Category: Pharma Industry
A federal appellate court in Washington, D.C. is fast-tracking Novartis and Bristol Myers Squibb’s (BMS) appeal of a lower court [...] …
The Centers for Medicare and Medicaid Services (CMS) released new details this week to help stakeholders navigate the Inflation Reduction [...] …
Three major drugmakers recently announced refunds for 340B covered entities that purchased certain drugs in 2022 and 2023 following recalculations [...] …
In the final hour of Oklahoma’s 2025 legislative session, as the clock neared 11 p.m. on May 29, state legislators [...] …
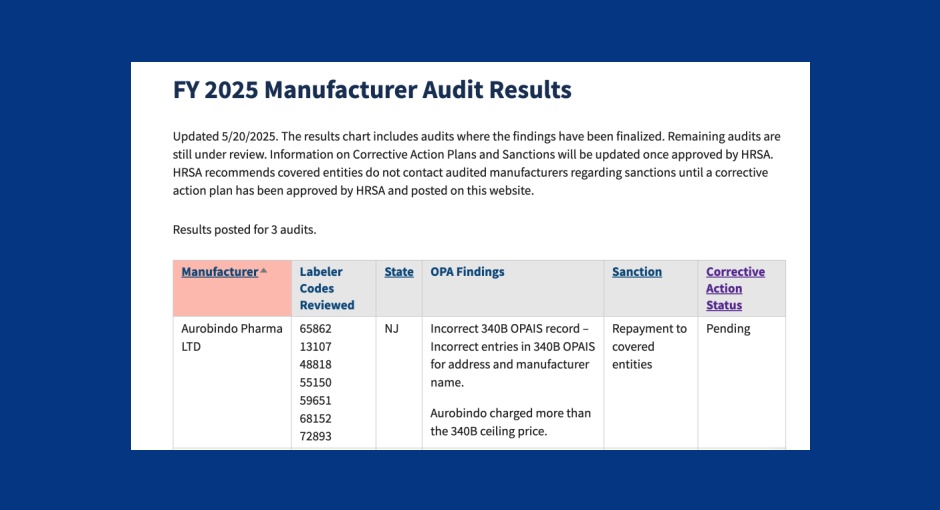
For the third straight time in 2025, a federal audit has found a drug manufacturer overcharged covered entities for 340B [...] …
A recent drug-industry-funded study attributes the 340B program’s recent growth to increased utilization—not rising drug prices. The May 21 study, [...] …
Novartis and Bristol Myers Squibb (BMS) are asking a federal appeals court to expedite their challenge to a recent ruling [...] …
Pharmaceutical giant Amgen recently announced it will exempt Tennessee providers from its contract pharmacy restrictions despite a carve out in [...] …
SpecGx, a generic subsidiary of Mallinckrodt Pharmaceuticals, will refund 340B covered entities that purchased addiction treatment medication Methadose for above [...] …