Category: Pharma Industry
ASAP 340B, the nonprofit group that the brand drug industry and health centers formed to help advance the two sides’ joint plan to remake the 340B program, held its first webinar yesterday to describe its policy principles and call for
…Drug manufacturer Boehringer Ingelheim announced this morning that, starting Aug. 1, all covered entity types will be subject to its conditions on 340B pricing when entities use contract pharmacies to dispense BI products. Until now, its conditions have applied to
…Ryan White Clinics for 340B Access yesterday criticized drug manufacturer Gilead Sciences for sponsoring a study that said it is unclear if patients of Ryan White clinics and other 340B grantee covered entities benefit from the drug margin these entities
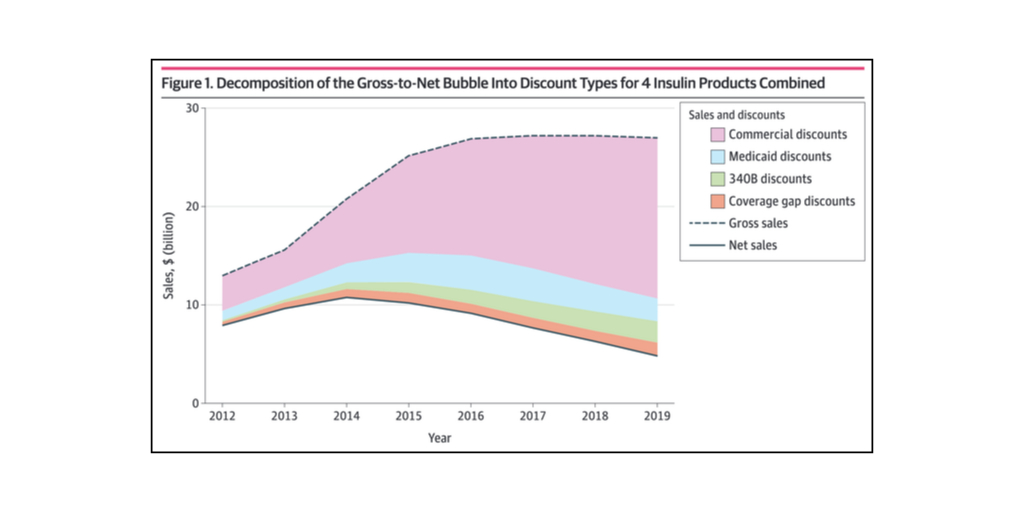
…Drug manufacturers often observe that a growing share of their take from gross sales goes to middlemen and others in the form of rebates and discounts. A term has been coined to describe the phenomenon: the gross-to-net bubble (the difference
…Biopharmaceutical manufacturer Exelixis said today it is extending its conditions on 340B pricing to contract pharmacies that are wholly owned or under common ownership with a hospital covered entity. The change is effective June 26.
Exelixis announced the change in
…Drug manufacturer Teva today became the 23rd drug manufacturer to impose conditions on 340B pricing involving delivery to contract pharmacies.
Covered entities reported getting Teva’s notice by email around 1:00 p.m. Eastern. It said effective July 5:
Federal Medicare drug price negotiation “is tantamount to extortion” and must be struck down as unconstitutional, drug manufacturer Merck told a federal district court yesterday.
Merck on Tuesday became the first drug maker to sue to stop the federal government
…The U.S. Health Resources and Services Administration yesterday posted notices of refunds for 340B overcharges from drug manufacturers Lilly, Novo Nordisk, and Neurocrine and a notice from Amneal about product NDC changes.
Lilly
Lilly’s May 30 notice covers purchases
…