The fallout continues from drug makers and health centers’ decision to work together to change the 340B program, with the drug industry giving its first interview about the deal to 340B Report on Friday and several hospital groups jointly blasting
…Category: Pharma Industry
Biopharmaceutical manufacturer Amgen this afternoon significantly expanded its conditions on 340B pricing when hospitals use contract pharmacies.
Johnson & Johnson announced a similar expansion of its 340B contract pharmacy limits last month. Hospital groups slammed J&J’s new restrictions.
Amgen’s
…A large HIV/AIDS health care group, a Democratic 340B provider ally in Congress, and three national hospital groups lambasted community health centers and drug makers’ historic announcement yesterday that they are working together to remake the 340B program. Other 340B
…Breaking News
Major Drug Industry and Health Center Groups Unite to Reform 340B for Patients and “True” Safety-Net Providers
Pharmaceutical Research and Manufacturers of America, the National Association of Community Health Centers, and the National Hispanic Medical Association this morning jointly issued a 10-point plan and created a nonprofit group to ensure that the 340B program “benefits patients and
…Generic and specialty drug manufacturer Amneal Pharmaceuticals is offering refunds for 340B overcharges on an 18-page-long list of products for purchases between Q3 2019 and Q3 2022.
The U.S. Health Resources and Services Administration posted New Jersey-based Amneal’s notice to
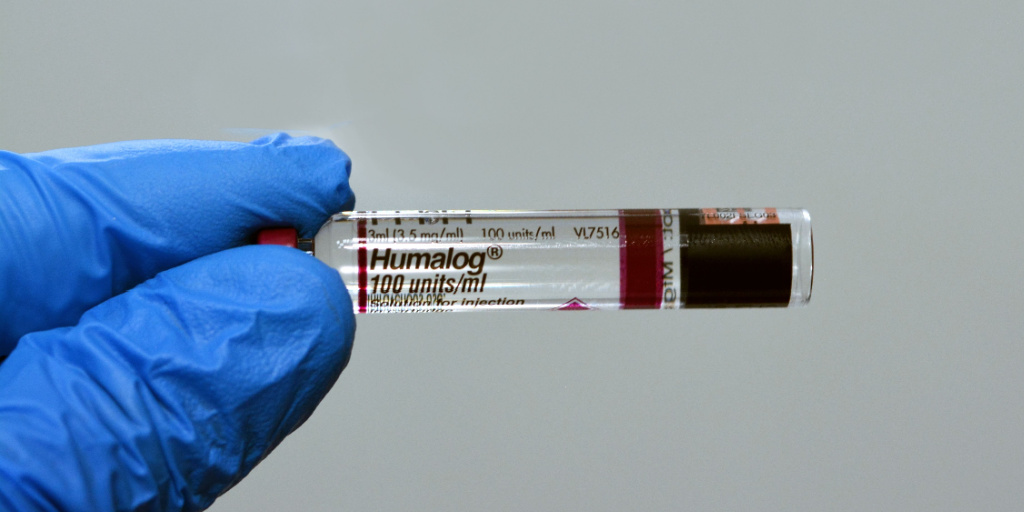
…Drug manufacturer Lilly’s announcement last week that it is slashing the price of Humalog, its most commonly prescribed insulin, may have been less about altruism and more about avoiding exposure to bigger Medicaid rebates, a prominent drug pricing expert says.
…Generic drug manufacturer Zydus Pharmaceuticals USA said in a recent public notice it will pay refunds for 340B overcharges that occurred mostly in the first two quarters of 2022. The products include Zydus’ equivalents of the widely prescribed antibiotic Zithromax,
…Associations representing community health centers and brand drug manufacturers are jointly developing 340B policy proposals for Congress to consider, hospital group 340B Health told its members yesterday.
The National Association of Community Health Centers said yesterday that it had no
…Johnson & Johnson, the world’s biggest drug company, yesterday announced a major expansion of the drug industry’s campaign to set limits on 340B drug sales.
On March 7, it is poised to become the first drug maker to place a
…Pharmaceutical manufacturer GlaxoSmithKline said last week it would pay refunds for 340B overcharges occurring in the first quarter of 2021, including overcharges for blockbuster respiratory drugs Trelegy and Nucala.
The refund notice posted Feb. 2 on the U.S. Health
…