The U.S. Food and Drug Administration (FDA) last week approved the first gene therapy to treat adults with hemophilia B, a genetic bleeding disorder resulting from missing or insufficient levels of a protein needed to make blood clot. About 15%
…Category: Pharma Industry
340B hospitals use biosimilar drugs less than other hospitals, which may expose patients to higher out-of-pocket costs, a drug industry funded study concludes.
Pharmaceutical Research and Manufacturers of America released the study last week. Healthcare consulting firm Milliman did the research for
…The U.S. Health Resources and Services Administration has ordered Pennsylvania-based generic drug manufacturer KVK Tech to repay 340B covered entities for program violations found during an audit.
HRSA posted its audit findings Nov. 15. “KVK-Tech failed to refund covered entities for
…The havoc caused by Twitter’s new policy on user identity verification struck drug manufacturer Eli Lilly last week when a counterfeit account bearing one of Twitter’s new $8 checkmarks tweeted, “We are excited to announce insulin is free now.”
The
…Drug manufacturers GlaxoSmithKline and Purdue Pharma have announced refunds for overcharging 340B covered entities, GSK for the fourth time and Purdue for the second time this year.
The U.S. Health Resources and Services Administration (HRSA) recently posted the companies’ notices
…Getting 340B pharmacy data to study trends and public health impacts “is like trying to catch a will-o’-the-wisp,” a former senior Trump administration health care official said in a commentary last week in the life sciences news publication STAT.
“The
…Now that a federal appeals court in Washington, D.C., has heard arguments in Novartis and United Therapeutics’ 340B contract pharmacy lawsuits, the scene shifts to Chicago, where an appeals court will hear arguments Monday morning in Eli Lilly’s companion case.
…AIDS Healthcare Foundation (AHF) members are staging a weeklong, twice-daily series of protests at drug maker Gilead Science’s Northern California headquarters to protest the company’s policy to impose conditions on 340B providers that use contract pharmacies to dispense 340B-priced drugs.
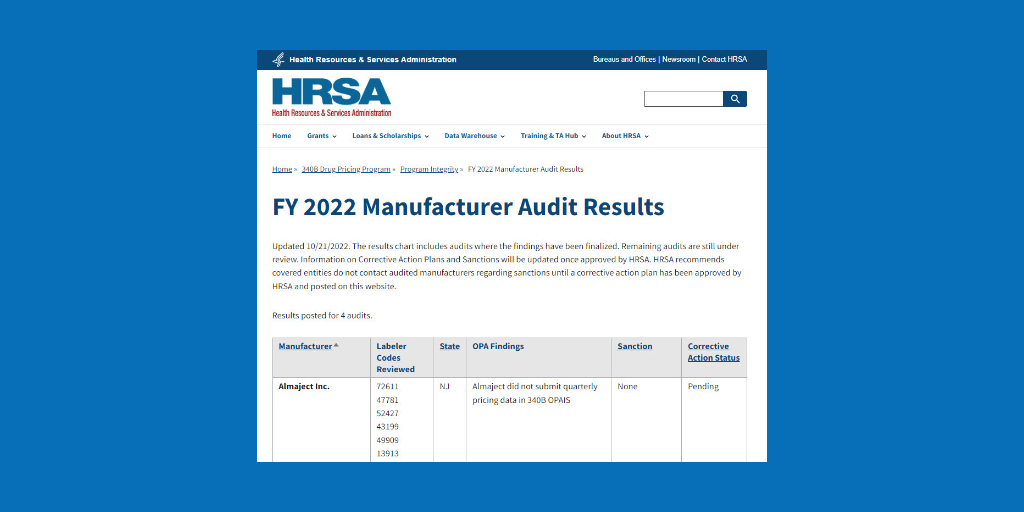
…The U.S. Health Resources and Services Administration (HRSA) has cited Almaject, a New Jersey-based maker of generic injectables, with failing to submit quarterly pricing data into the 340B Office of Pharmacy Affairs Information System, although the agency did not impose
…The U.S. Health Resources and Services Administration yesterday referred drug manufacturer Merck to the Department of Health and Human Services Office of Inspector General for possible imposition of civil fines over Merck’s 340B contract pharmacy restrictions.
Public Health Services Lt.
…