Nonprofit drug manufacturer Civica announced this morning that it plans to manufacture and distribute insulins that, once approved, will be available to people with diabetes at no more than $30 per vial and no more than $55 for a box
…Category: Pharma Industry
Thirteen congressional Democrats—eight senators and five representatives—asked Pharmaceutical Research and Manufacturers of America (PhRMA) to respond by March 8 to questions about “rapid price hikes affecting the vast majority of popular brand name drugs.”
Sens. Elizabeth Warren (Mass.) and Amy
…340B provider and drug industry stakeholders joined yesterday in congratulating U.S. Public Health Service Lt. Cmdr. Emeka Egwim on his selection as Director of the U.S. Office of Pharmacy Affairs, the federal agency that runs the 340B drug pricing program.
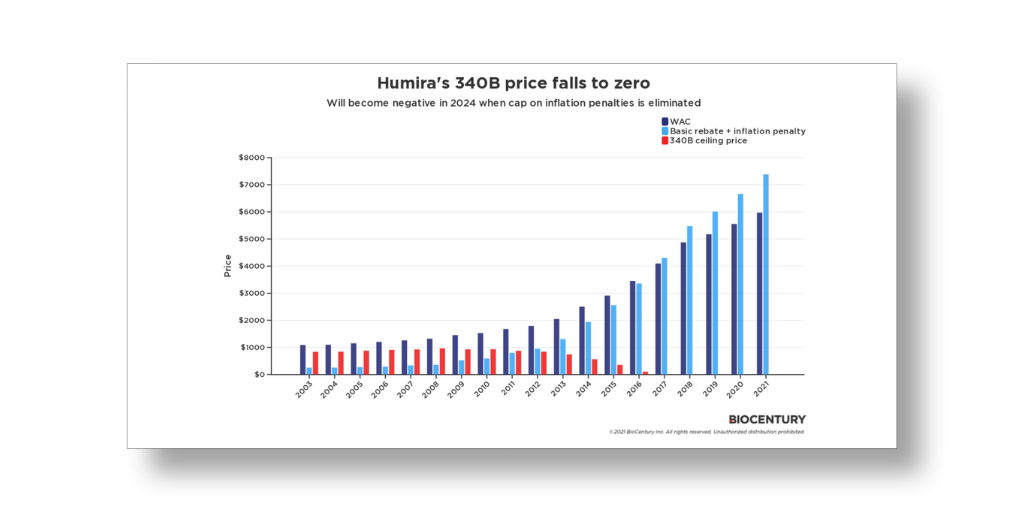
…In less than two years, brand drug companies that now must sell their products to 340B covered entities for a penny due to a history of price increases above inflation may have to start paying entities for their drugs instead
…Drug manufacturer Merck is providing 340B covered entities with refunds for overcharges on 17 NDCs for purchases between Jan. 1 and March 31, 2019, the company says in a new public notice on the U.S. Health Resources and Services
…In a Win for Health Centers, 340B ADR Panel Says Proceedings Against AstraZeneca & Sanofi Must Go On
A 340B administrative dispute resolution (ADR) panel decided late last week that it will not stop proceedings in the National Association of Community Health Centers’ (NACHC) dispute-resolution petition over drug manufacturers AstraZeneca and Sanofi’s conditions on 340B pricing when covered
…Drug manufacturer Lilly indicated late last week that it reserves the right to seek additional compensation for its products from 340B covered entities if it learns later that it charged them less than what the company perceives the 340B statute
…America’s Essential Hospitals and 340B Health yesterday denounced GlaxoSmithKline’s (GSK) new restrictions on 340B pricing when hospitals use contract pharmacies. Pharmaceutical Research and Manufacturers of America (PhRMA) said this morning it will keep pushing for “much needed changes” to
…UPDATE Monday, Feb. 14, 2022, 1:30 p.m. EST—The U.S. Health Resources and Services Administration (HRSA) said, “We are reviewing GSK’s policy and will evaluate next steps as needed.”
Drug manufacturer GlaxoSmithKline (GSK) told 340B covered entities this morning that effective
…