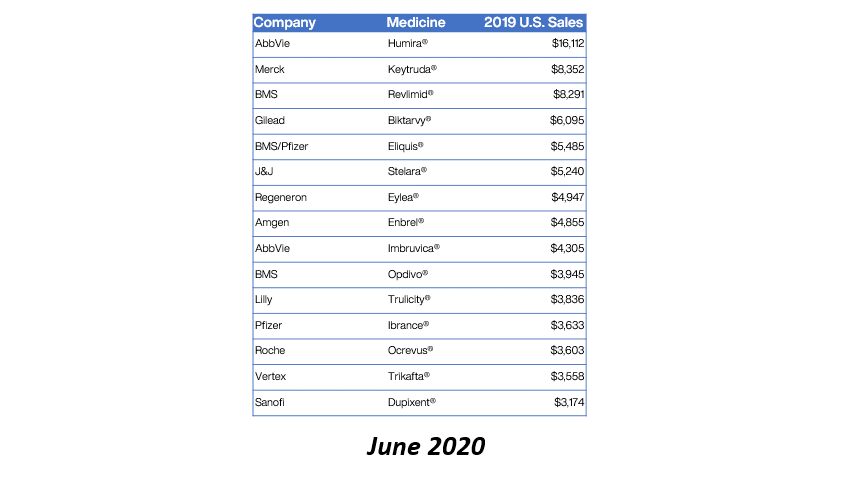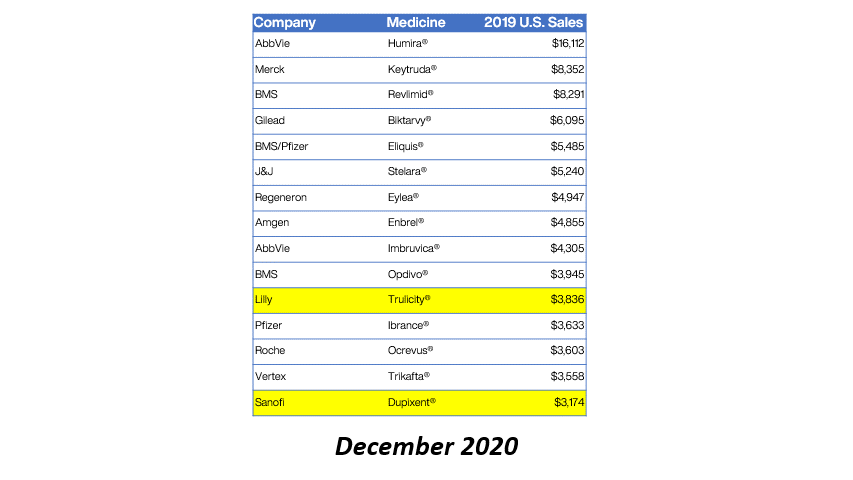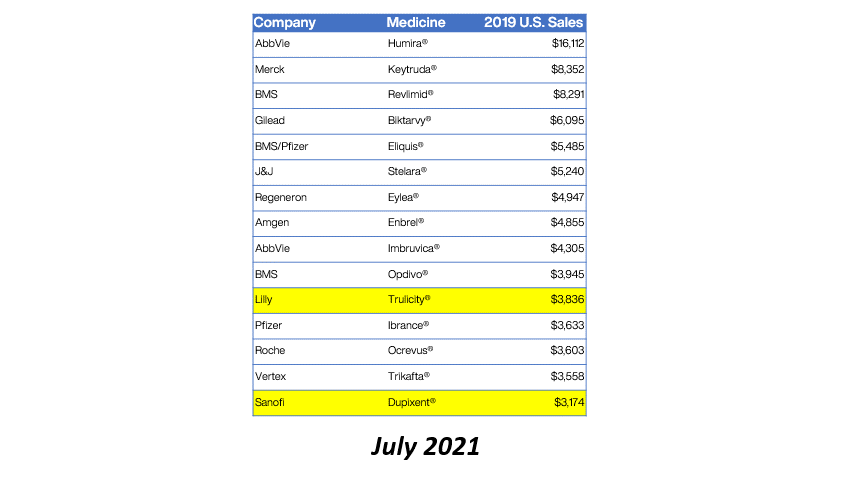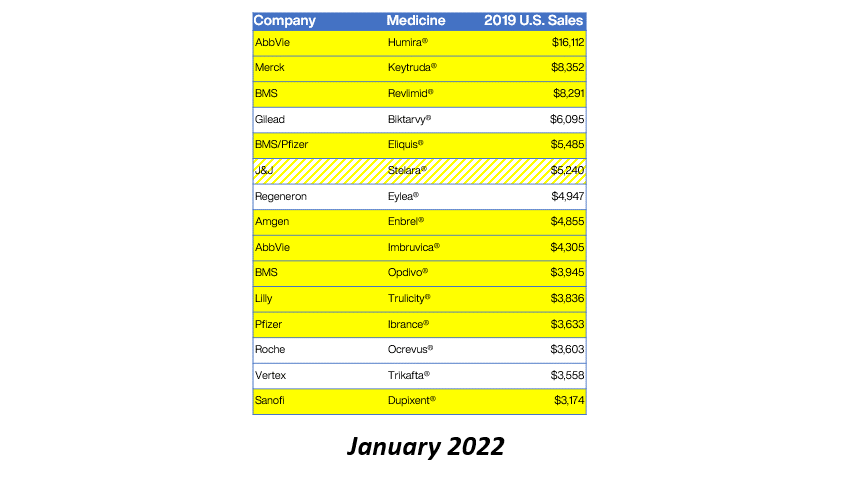
Drug manufacturer GlaxoSmithKline is providing refunds for charges above 340B ceiling prices on nine NDCs for purchases during Q1 2020.
GSK posted a public notice about the repayments on the U.S. Health Resources and Services Administration web site this
…