The U.S. Centers for Medicare & Medicaid Services wants to make Medicaid managed care organizations use Medicaid-specific codes and group numbers on beneficiary insurance cards to help states, MCOs, and 340B covered entities avoid invoicing manufacturers for prohibited duplicate 340B
…Category: Regulatory
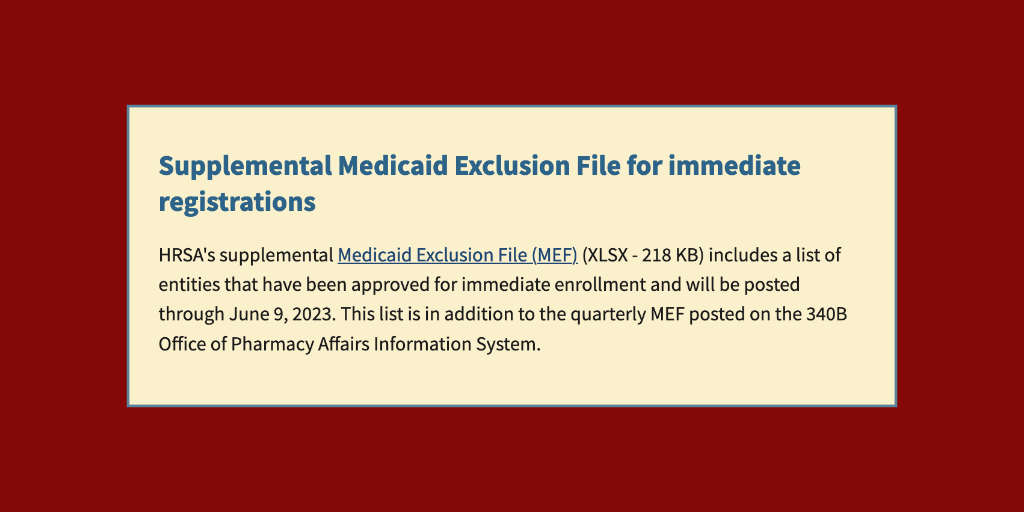
Federal 340B program officials confirmed last week that providers granted immediate 340B enrollment under a COVID-19 public health emergency flexibility are not losing their 340B eligibility and will not need to re-enroll now that the PHE is over.
The U.S.
…Drug manufacturers Amgen and GlaxoSmithKline have notified 340B covered entities about refunds for overcharges, Amgen for sales in 2020 and GSK for sales in 2021.
The U.S. Health Resources and Services Administration posted both companies’ notices on its website yesterday.
…Correspondence in April and May 2020 between the 340B prime vendor and a law firm sheds more light on the start of a policy that the federal government ended abruptly last week that let hospitals start using 340B drugs at
…Breaking News
HRSA and Prime Vendor Yank FAQs About 340B Use in Hospital Child Sites, and GAO Issues a New 340B Study
The U.S. Health Resources and Services Administration midday yesterday and the 340B prime vendor Apexus this morning, in connection with the end of the COVID-19 public health emergency late last night, withdrew mostly identical 340B program FAQs about use of
…Drug manufacturer Janssen Pharmaceuticals said last week it would pay refunds for overcharges on 340B drugs purchased in the second quarter of 2020, including top-selling psoriasis drug Stelara.
Janssen, part of Johnson & Johnson, said it owes or may owe
…Editor’s note: New information from HRSA and Apexus obtained as we were going to press appears at the end of this article.
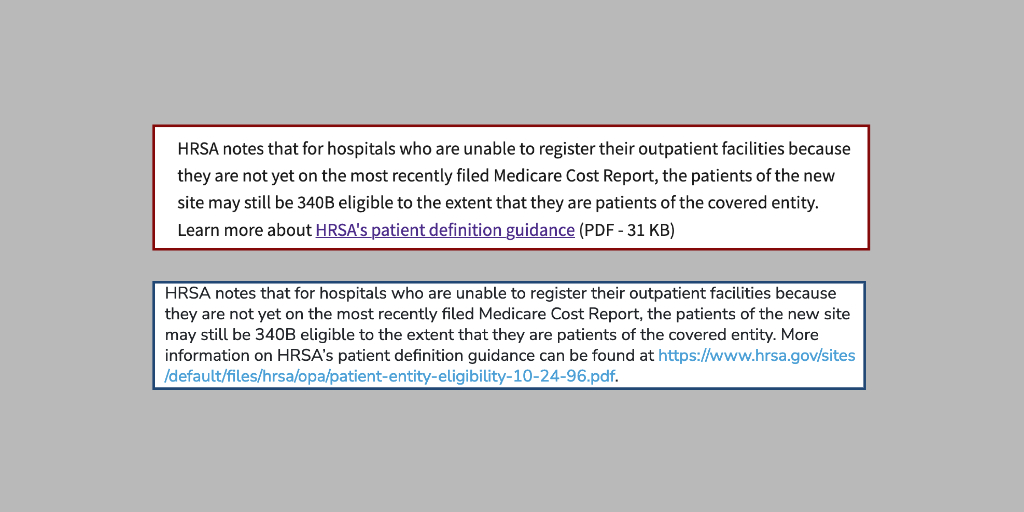
Hospital representatives said they were floored to learn yesterday that a June 2020 federal 340B patient eligibility policy
…Generic drug manufacturers Lifestar Pharma and Chartwell Pharmaceuticals may owe refunds to 340B covered entities for overcharges on drugs purchased from the companies in the fourth quarter of 2022, according to recent notices posted on the U.S. Health Resources and
…The U.S. Health Resources and Services Administration said late Friday that when the COVID-19 public health emergency ends on Thursday, so too will a nearly three-year-old policy clarification that lets hospitals under certain conditions dispense 340B drugs at offsite outpatient
…Certain flexibilities to the 340B program allowed under the COVID-19 public health emergency will expire May 11 alongside the PHE, the U.S. Health Resources and Services Administration announced Monday.
The update, which HRSA posted prominently on the Office of Pharmacy
…