Last September, the U.S. Health Resources and Services Administration (HRSA) published a drug manufacturers’ notice to 340B covered entities that it was limiting distribution of a medicine that alleviates a side effect of HIV/AIDS treatment.
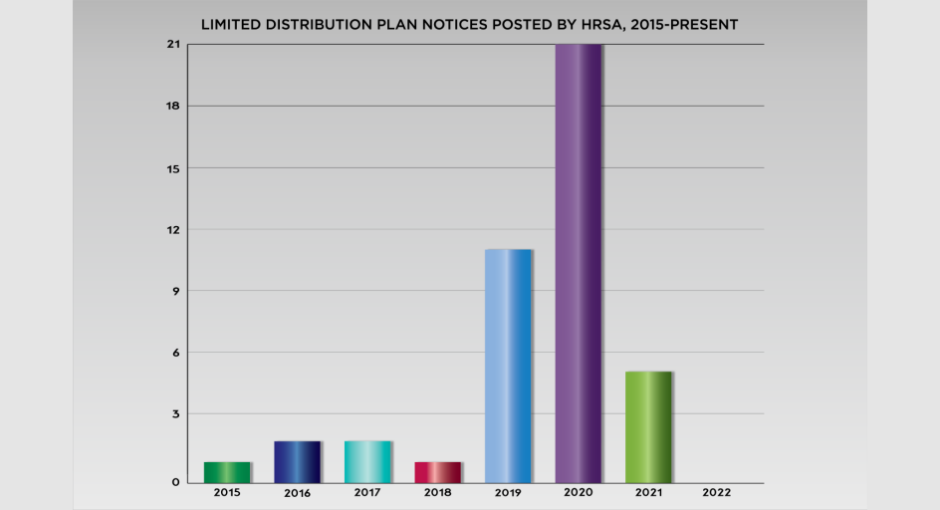
It was the fifth limited distribution plan notice that HRSA posted on the Office of Pharmacy Affairs (OPA) website last year and the 43rd since 2008, the farthest back that publicly available HRSA data on the subject go.
HRSA hasn’t posted another one since. And no one outside of HRSA appears to know why.
Several manufacturers have asked HRSA to post limited distribution plan notices to covered entities since 2021, but their notices remain unposted and HRSA has not responded to their inquiries, a person with direct knowledge of the requests said. The drugs involved, the distribution limits imposed, and the manufacturers’ stated reasons for the limits are unknown.
HRSA has acknowledged but not yet responded to multiple requests for comment about the absence of new limited distribution plan notices.
HRSA posted the first notice from a drug manufacturer to 340B entities that it was limiting distribution of a drug for a reason other than a drug shortage in 2015. There were two such notices each in 2016 and 2017, then one in 2018. The number jumped to 11 in 2019, and jumped again to 21 in 2020. Then it dropped back to five through the first three quarters of 2021. There have been none since.
340B stakeholders have two theories about why there have been no new limited distribution notices posted on the OPA site since last September.
One is that the pause is linked to the multiple lawsuits over drug manufacturers’ decisions to put conditions on 340B pricing when entities use contract pharmacies.
The other is that HRSA might be responding to years of complaints from entities over allegations that some manufacturers’ distribution limits are less about protecting the safety of patients and others than they are about padding the manufacturers’ profits.
HRSA’s Requirements and Expectations
Nearly all drug company notices to 340B entities posted on OPA’s webpage fall into one of three categories: (1) notices about refunds for overcharges, (2) notices about allocation systems when there is a drug shortage, and (3) notices about limited distribution plans. HRSA set its requirements and expectations for manufacturers about these notices in a 2017 regulation and a 2012 guidance document.
Regarding overcharges, HRSA’s 2017 340B ceiling price and manufacturer civil monetary penalties (CMP) regulation requires manufacturers to give affected entities notice only when they are refunding the difference between the estimated 340B price of a newly launched drug and the drug’s actual 340B price calculated one year later.
Commenters had asked HRSA to clarify whether its refund policy for new drugs would also apply to overcharges identified during price restatements. Commenters also asked HRSA to codify a formal refund procedure in regulation.
“The refund requirements as set forth in this final rule apply as it relates to new drug price estimations,” HRSA answered in the rule’s preamble. “Specific procedures for refunds are outside the authority of this final rule and will be addressed in future guidance.”
The ceiling price/CMP regulation does include a section warning manufacturers that they could be liable for civil monetary penalties for a variety of overcharges including restatements. Drug manufacturers have regularly posted notices of overcharges for various instances of overcharges. Jason Reddish, a partner at Feldesman Tucker who represents 340B providers and has challenged alleged overcharges, said drug manufacturers likely ask HRSA to post their 340B refund notices as a precaution. “Failing to notify covered entities of a price discrepancy could be grounds for finding that they ‘knowingly and intentionally’ overcharged for a 340B drug, which would place them at jeopardy for CMPs,” he said.
In fact, since October 2021, OPA has posted nothing but manufacturer refund notices on its Manufacturer Notices to Covered Entities webpage. There have been 25 so far this year—a record high with a more than a full calendar quarter still to go. The previous record was 18 refund notices last year. The most recent one was just this week.
Regarding drug shortages, HRSA’s 2012 340B program guidance entitled Clarification of Non-Discrimination Policy asks but does not require drug makers to notify OPA when they develop “alternate allocation procedures” for drugs in short supply. The guidance says OPA will publish submitted plans on its website.
“Although prior notification by manufacturers is not currently required, HRSA believes that voluntarily providing OPA with timely notification will benefit manufacturers as well as covered entities by reducing the chance for misunderstandings about the requirements of the 340B program and lessen the potential for disputes,” the guidance said.
The last notice on the OPA website about a manufacturer’s supply allocation system was last October. It was Bristol Myers Squibb’s announcement that it had lifted purchasing limits on a leukemia drug in place since 2020 due to supply constraints. There were four such notices posted in 2021 and 2020 each and five posted 2019, but none in the preceding four years and no more than one a year in the seven years before that.
How HRSA treats manufacturer notices to entities about limited distribution plans springs from a combination of the 2017 regulation and the 2012 guidance.
Even though HRSA’s 2012 non-discrimination guidance was silent about manufacturers’ limited distribution plans, HRSA’s 2017 ceiling price and manufacturer CMP regulation cited the guidance in explaining how HRSA would assess manufacturers’ 340B compliance when they implement limited distribution plans or sell drugs through specialty pharmacies.
“Manufacturers may continue to develop limited distribution procedures provided that those arrangements follow HHS established policy,” HRSA said in the regulation. “HHS will take into consideration whether a manufacturer has submitted an alternate allocation plan to HHS when a manufacturer is being investigated for a possible overcharge, whether this plan is compliant with the 340B non-discrimination policy, and whether the manufacturer is following its plan.”
Limited Distribution Notices and the Fight Over 340B Contract Pharmacy Use
Attorneys for manufacturers and 340B entities speaking on background agreed that HRSA might have stopped posting all drugmakers’ limited distribution plan notices due to the litigation over 340B contract pharmacy—even those notices unrelated to contract pharmacy.
Eli Lilly was the first drug manufacturer to announce it was limiting distribution of a 340B covered outpatient drug—three formulations of Cialis—to entities’ in-house pharmacies only or to just one contract pharmacy if the entity did not have its own pharmacy. Lilly submitted a limited distribution plan notice to covered entities about its new policy to HRSA to be posted on the OPA site. HRSA posted Lilly’s notice on the OPA website in early July 2021, where it remains to this day.
Two months later when Lilly announced its was expanding its limits on 340B drugs sales to all its covered outpatient drugs, it prepared another limited distribution plan notice to covered entities to announce the change. Its format matches the first notice. It is not clear from the record in Lilly’s subsequent lawsuit against HRSA if the company ever submitted the second notice to the agency to be posted on the OPA site. If it did, HRSA did not post it. Covered entities reported receiving the second notice from Lilly by email.
It is not known if any of the 17 other manufacturers that impose conditions on 340B pricing when covered entities use contract pharmacies asked HRSA to post their announcements on the OPA website. If any did, HRSA has not published them.
“It is disappointing that HRSA seems to have suspended the publication of limited distribution plan notices,” said John Shakow, a partner at King & Spalding who represents drug manufacturers. “Manufacturers work hard to ensure that covered entities can access 340B pricing for drugs that, for clinical and patient care reasons, can’t be made available through the standard wholesaler channel. The notices are manufacturers’ good faith attempts to communicate important information to covered entities. Hopefully, the agency will resume publication of LDP notices soon.”
Taking a Harder Line on LDPs?
The other theory about why HRSA hasn’t posted a limited distribution plan notice in almost a year is that HRSA is taking a harder line on them.
The first manufacturer to post such a notice was Celgene in 2015. As described in a six-part 340B Report investigation last year, a former Celgene vice president alleged in a federal whistleblower lawsuit that Celgene did not limit distribution of three highly profitable cancer drugs for safety reasons as it said, but instead “to deny access to the drugs at the [340B] ceiling price.” The whistleblower estimated that Celgene made about $298,000,000 in profit in 2018 alone by not paying 340B ceiling prices on Revlimid, Pomalyst, and Thalomid.
In 2017, HRSA told Celgene in response to a hospital’s complaint that the company had to ensure that covered entities could buy the three cancer drugs at the 340B ceiling price through the limited distribution network.
HRSA, however, reportedly “never followed up” on its letter to Celgene. Then in 2018, for reasons that have never been explained, the U.S. Justice Department declined to intervene in the whistleblower’s lawsuit, and the complaint against the company was withdrawn.
In December 2020, 28 state attorneys general urged the U.S. Health and Human Services Department to address drug manufacturers’ denials of 340B pricing involving the contract pharmacy program. HHS Secretary Xavier Becerra was California Attorney General at the time and was a driving force behind the letter.
“Notwithstanding clear legal requirements, some drug manufacturers have brazenly ceased providing 340B pricing to covered entities using contract pharmacies and others have unilaterally imposed conditions on 340B pricing,” the letter said, specifically citing Celgene’s 2015 limited distribution notice for Revlimid, Pomalyst, and Thalomid.
“It looks to me like HRSA is probing these notices more deeply, not just rubber stamping them, and asking whether they might have a discriminatory impact,” said William von Oehsen, a partner at Powers Law who represents covered entities and who has also challenged alleged drug company overcharges. “That might be the reason why they aren’t getting published. Manufacturers probably don’t want to answer those questions.”