An Alabama health system and a large health insurer appear to have reached a settlement over allegations that the insurer’s [...] …
Category: Regulatory
A federal judge in Washington, D.C. has rejected a University of Alabama Health System lawsuit seeking interest on Medicare’s lump [...] …
Trade groups representing 340B hospitals and hospital pharmacists recently urged the Health Resources and Services Administration (HRSA) to extend the [...] …
Two major drugmakers recently announced additional 340B contract pharmacy restriction exemptions for states that have enacted new contract pharmacy access [...] …
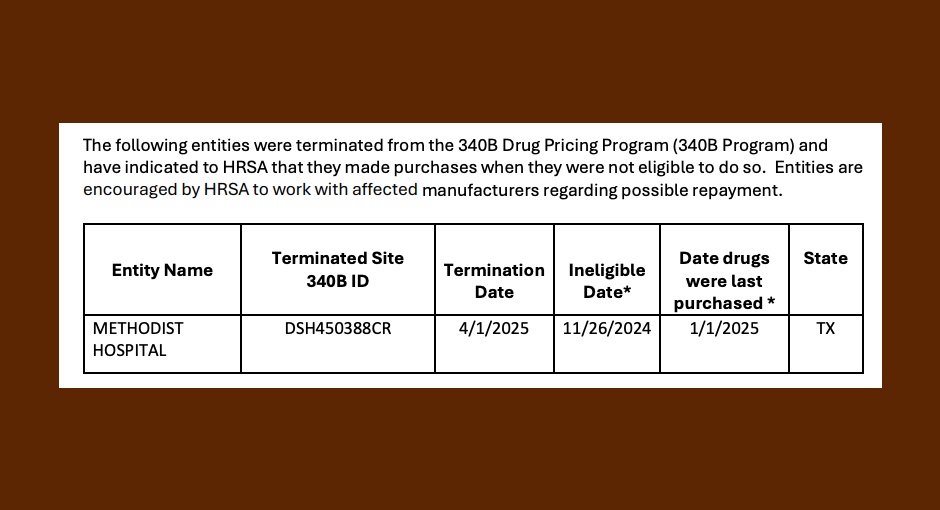
The Health Resources and Services Administration (HRSA) recently announced that a Texas health system’s location has been removed from the [...] …
Sexually transmitted disease (STD) clinics and other 340B covered entities must submit new or more complete information to enroll in [...] …
The U.S. Department of Health and Human Services (HHS) touted the agency’s recently announced plan to pilot a 340B rebate [...] …
President Donald Trump is urging more than a dozen leading pharmaceutical manufacturers to begin implementing his proposed “most-favored nation” (MFN) [...] …
340B covered entities and drugmakers offered competing criticisms Thursday of the Health Resources and Services Administration’s (HRSA) newly released 340B [...] …
The Health Resources and Services Administration (HRSA) today unveiled its highly anticipated 340B rebate guidance, proposing a pilot program that [...] …