A national 340B community health center organization, which has faced criticism for its partnership with a powerful drug industry trade [...] …
Category: Regulatory
A major North Carolina health system has sued insurers Aetna Health and Humana for allegedly underpaying for 340B drugs purchased [...] …
A federal appellate court in Washington, D.C. is fast-tracking Novartis and Bristol Myers Squibb’s (BMS) appeal of a lower court [...] …
The Centers for Medicare and Medicaid Services (CMS) released new details this week to help stakeholders navigate the Inflation Reduction [...] …
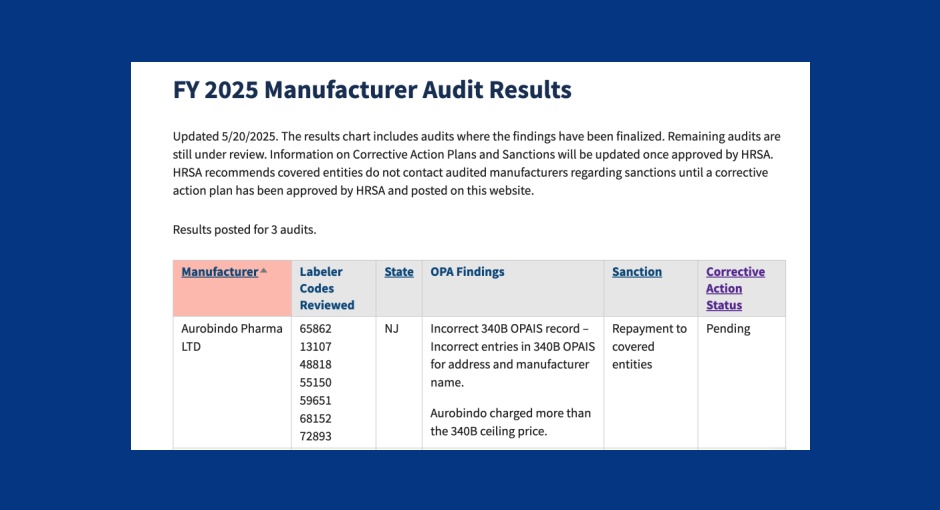
For the third straight time in 2025, a federal audit has found a drug manufacturer overcharged covered entities for 340B [...] …
A major group purchasing organization (GPO) recently urged a Washington D.C. federal court to reject the federal government’s defense of [...] …
SpecGx, a generic subsidiary of Mallinckrodt Pharmaceuticals, will refund 340B covered entities that purchased addiction treatment medication Methadose for above [...] …
The Department of Health and Human Services (HHS) has submitted proposed 340B rebate guidance for regulatory review—but it is unclear [...] …
Breaking News
Trump’s Full 2026 Budget Request Would Shift 340B Program from HRSA to CMS, Maintain Current OPA Funding
President Donald Trump’s fiscal year 2026 budget request released yesterday calls for moving control of the 340B program away from [...] …
The first published decision under the revamped 340B Administrative Dispute Resolution (ADR) process sided with the drug industry, marking a [...] …