The government agency that oversees the 340B program will retain just over half of its staff in the event of [...] …
Category: Regulatory
Drugmakers Lilly and Novo Nordisk are giving covered entities refunds on some products due to 340B ceiling price adjustments, according [...] …
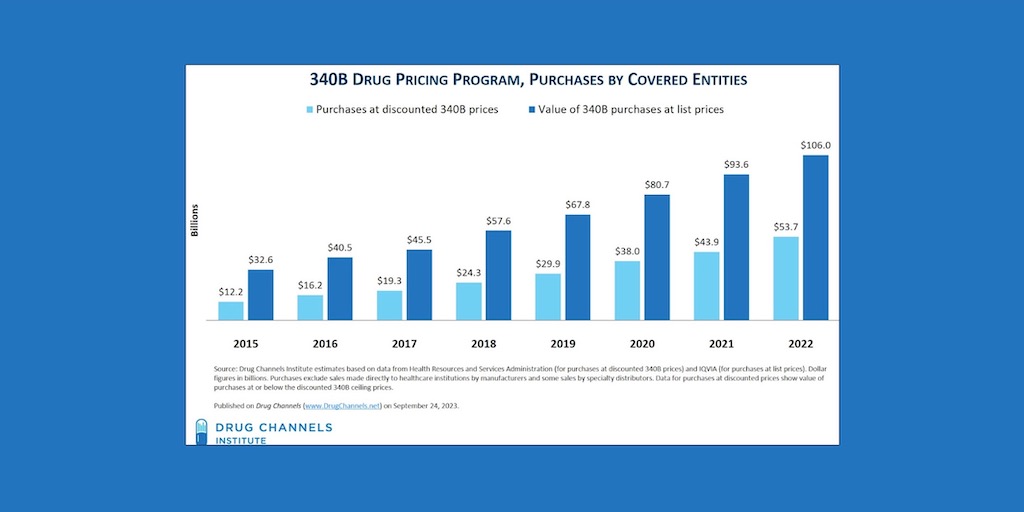
Drug purchases under the 340B program totaled $53.7 billion in 2022, or 22.3% more than the $43.9 billion in 2021, [...] …
Amid an increasing number of drugmaker restrictions and requirements on the use of contract pharmacies, 340B providers are trying a [...] …
The 340B program could more than double in size if the 340B patient definition expands, according to a recent report [...] …
A Senate committee rejected an effort to add new 340B hospital verification requirements as part of a sweeping healthcare funding [...] …
Nearly one-quarter of the 200 provider audits planned for this year were closed by regulators with no adverse findings, they [...] …
Republicans and Democrats battled over Medicare price negotiation provisions under the Inflation Reduction Act (IRA) during a contentious Sept. 20 [...] …
The AIDS Healthcare Foundation (AHF) recently filed an appeal to an April district court dismissal of its case against Apexus, [...] …
There is no evidence to support federal allegations that a South Carolina community health center illegally diverts 340B-purchased drugs to [...] …