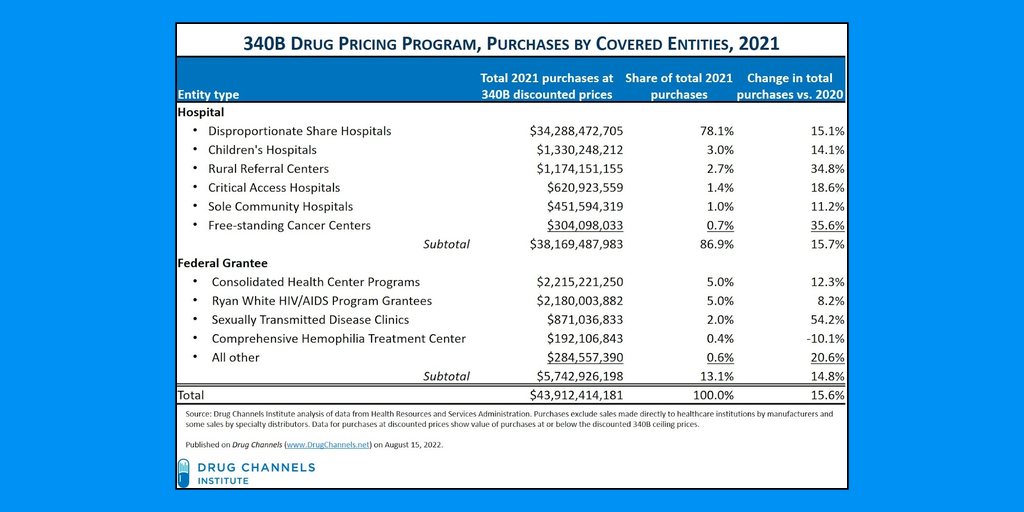
Total sales in the 340B drug pricing program reached $43.9 billion in 2021, a 15.6% increase over 2020 sales and more than 3.5 times above total sales in 2015 ($12.1 billion), according to federal data obtained by Drug Channels Institute
…Category: Regulatory
The U.S. Health Resources and Services Administration (HRSA) is letting eligible healthcare providers in flood-devastated Kentucky enroll in the 340B program immediately instead of making them wait until the next quarterly enrollment period in October.
U.S. Health and Human Services
…Purdue Pharma’s Avrio Health subsidiary is giving 340B covered entities refunds for overcharges on Betasept antiseptic surgical scrub for purchases spanning 16 years—from Q2 2005 through Q2 2021. That period covers more than half of the 340B program’s existence.
During
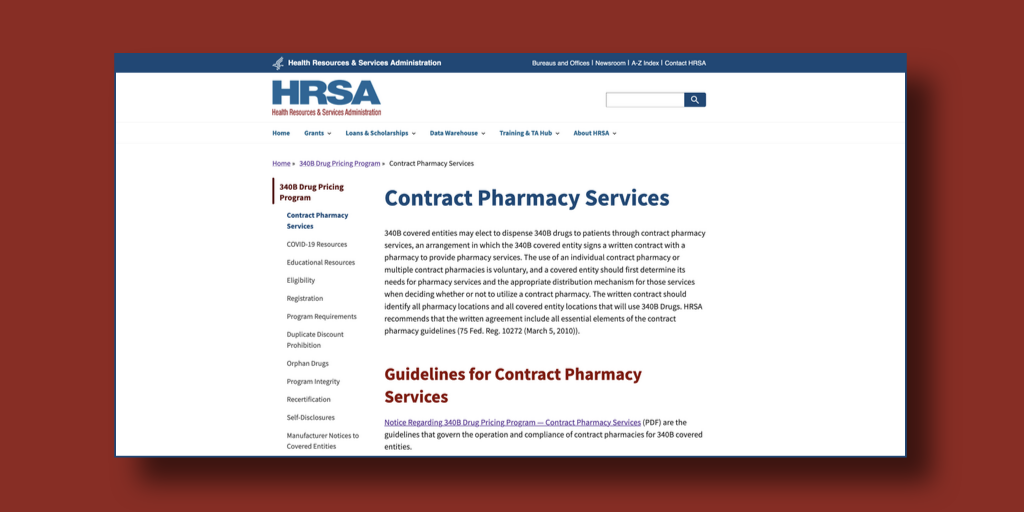
…The U.S. Health Resources and Services Administration overnight unveiled a new Contract Pharmacy Services page on the 340B program website that addresses requirements for covered entities but not for pharmaceutical manufacturers.
The webpage links to HRSA’s 2010 contract pharmacy final
…A Special Report From 340B Report:
A two-part series on the growing frustrations safety net providers face in restoring access to 340B pricing in the contract pharmacy setting. Part 1 provides important background on the challenges that covered entities face and
The Biden administration yesterday repealed Trump administration final rules that it said hindered the U.S. Health and Human Services Department’s (HHS) ability “to issue guidance, bring enforcement actions, and take other appropriate actions that advance the department’s mission.”
Under ex-President
…Groups that represent 340B hospitals praised the Biden administration’s decision Friday to end a deep cut in the hospitals’ Medicare Part B drug reimbursement in place since 2018. They urged the government to swiftly make 340B hospitals whole for their
…The U.S. Health Resources and Services Administration (HRSA) Office of Pharmacy Affairs (OPA), the unit within the Health and Human Services Department (HHS) that runs the 340B drug discount program, refreshed the OPA site late last week. This is the
…The U.S. Centers for Medicare and Medicaid Services “fully anticipate[s]” applying a Medicare Part B reimbursement rate next year of average sales price ASP plus 6% to drugs and biologicals purchased through the 340B program, CMS announced late this afternoon.
…