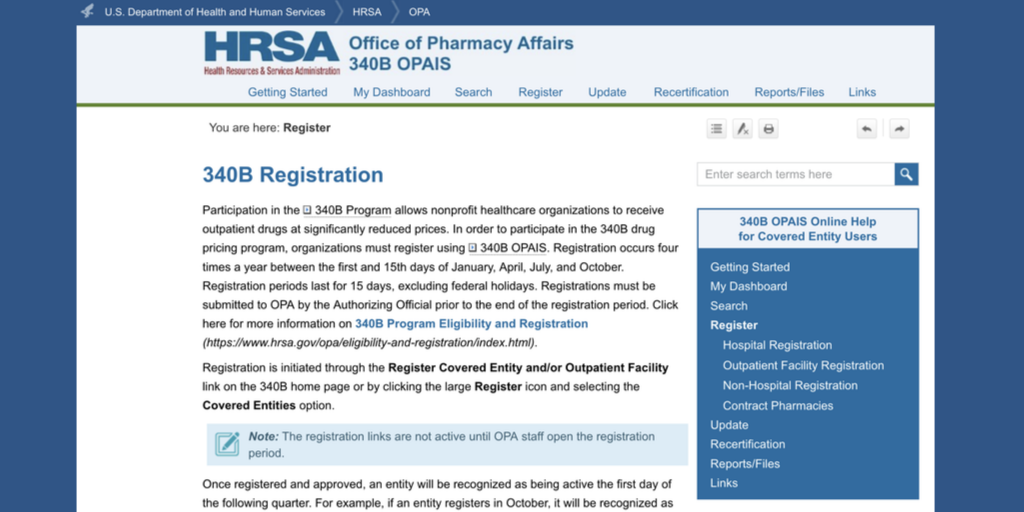
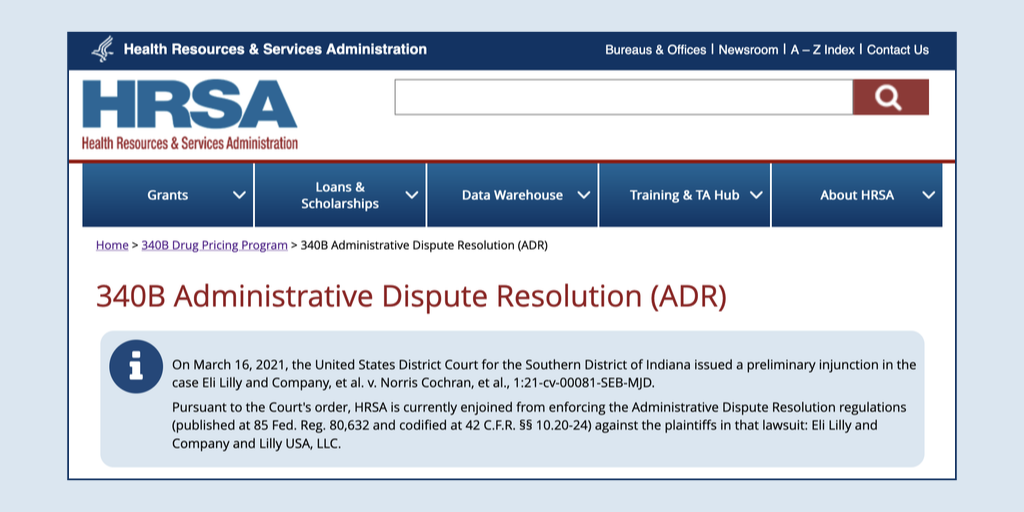
The U.S. Department of Health and Human Services issued guidance to retail pharmacies yesterday about their “unique role … in ensuring access to comprehensive reproductive health care services”—including filling prescriptions for drugs that can end or prevent pregnancy.
Mifepristone and
…