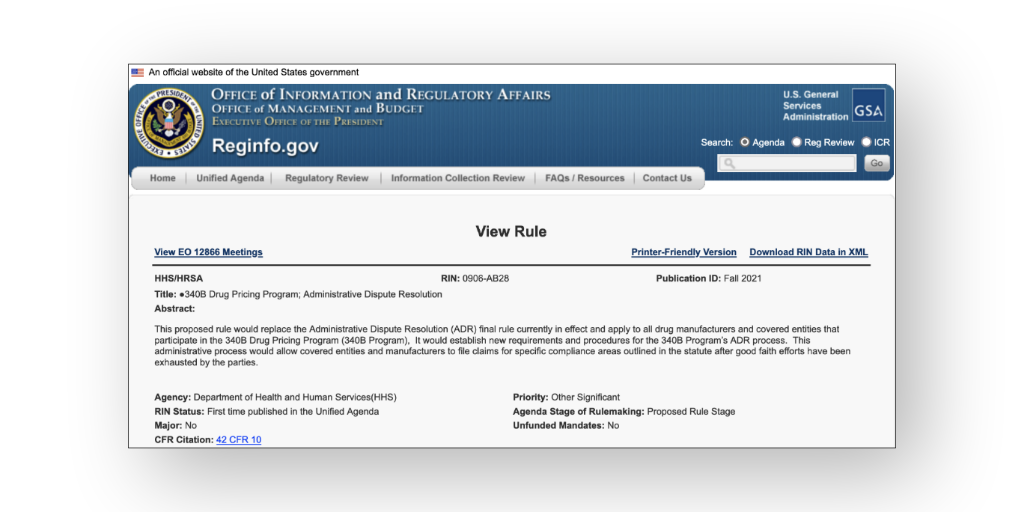
The American Hospital Association has asked U.S. Health and Human Services Secretary Xavier Becerra again to waive a requirement that it says is forcing hospitals out of the 340B program due to COVID-19-related changes in payer mix.
AHA President and
…