A federal judge in Sacramento, Calif., late yesterday declined to stop California Medicaid’s transfer of managed care drug coverage to Medicaid fee for service that took effect on Jan. 1. Health centers enrolled in the 340B drug pricing program pledged
…Category: Regulatory
The U.S. Health Resources and Services Administration (HRSA), the agency that runs the 340B drug pricing program, has a permanent leader again for the first time in almost a year.
Carole Johnson started in her position as HRSA administrator on
…The Biden administration is rescinding an unimplemented Trump-era regulation that would have pegged Medicare Part B drug reimbursement for 50 expensive, physician-administered drugs on the lowest price that drug manufacturers provide in similar countries.
The U.S. Centers for Medicare &
…The first 340B covered entity registration period of the year will be Jan. 1-18, 2022, for an effective start date of April 1, 2022.
Eligible hospitals, health centers, clinics, and their child sites and contract pharmacies registered during the period
…Just over 850 hospitals in the 340B program asked the federal government yesterday to appeal a judge’s Nov. 5 ruling that halted enforcement actions against two drug companies that deny 340B pricing when hospitals and others use contract pharmacies. They
…White House COVID-19 testing czar and long-time Democratic health policy expert Carole Johnson will become the next head of the U.S. Health Resources and Services Administration (HRSA) in January.
HRSA Acting Administrator Diana Espinosa announced Johnson’s selection this afternoon via
…As California’s Medicaid program moves toward implementing a fee-for-service payment structure for drug coverage, one of the primary foes of the transition has returned to court to obtain a temporary restraining order.
The new Medi-Cal Rx program is intended to
…Drug manufacturer Lilly said this morning it looks forward to reviewing the Biden administration’s proposed new 340B administrative dispute resolution (ADR) regulation when it becomes available “and hope[s] that it addresses the serious shortcomings of the current rule.”
“As Lilly
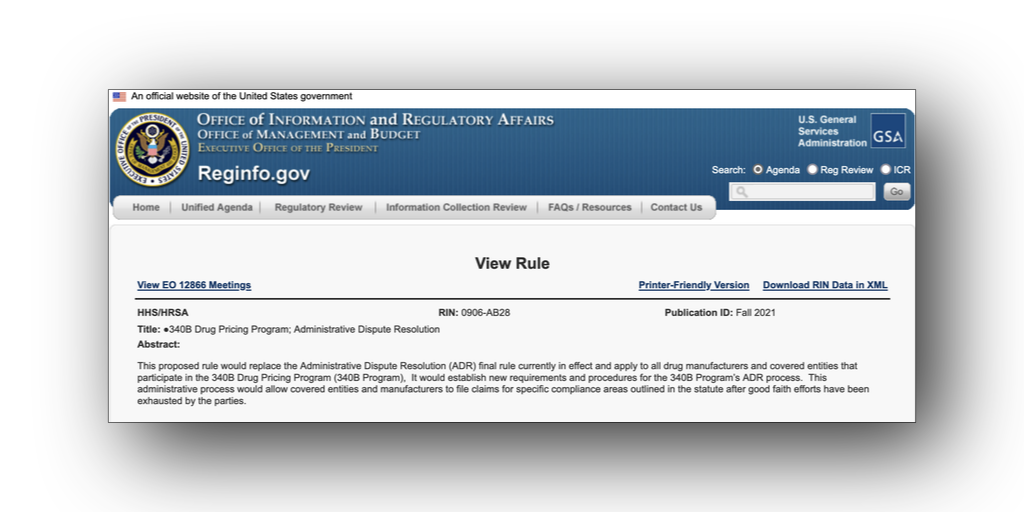
…The Biden administration plans to replace its 340B administrative dispute resolution (ADR) regulation with an entirely new rule that “better aligns with the president’s priorities on drug pricing [and] better reflects the current state of the 340B program.”
The administration
…