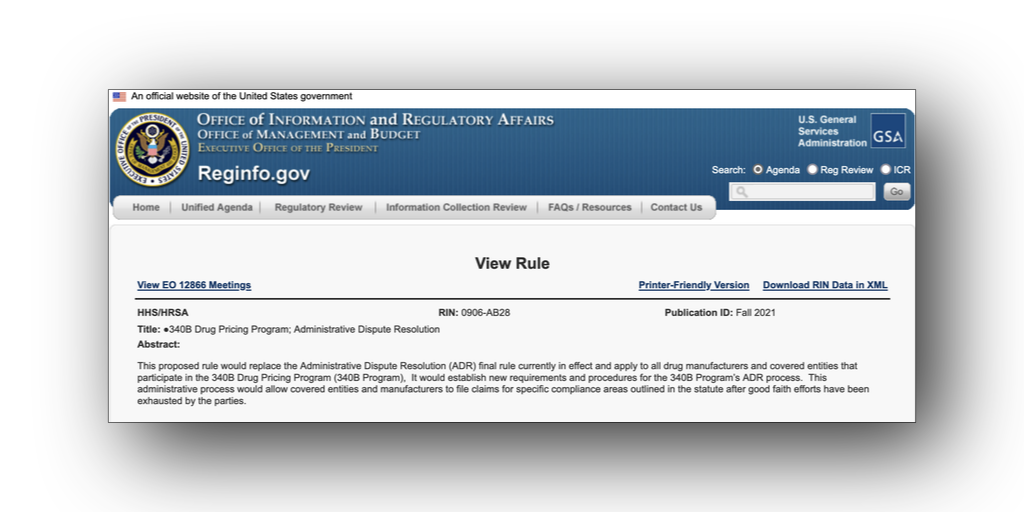
The Biden administration plans to replace its 340B administrative dispute resolution (ADR) regulation with an entirely new rule that “better aligns with the president’s priorities on drug pricing [and] better reflects the current state of the 340B program.”
The administration
…