An Arkansas Insurance Department hearing officer said last week she would decide by Oct. 13 whether to stay enforcement of a state law requiring drug manufacturers to honor 340B contract pharmacy arrangements pending resolution of a bevy of federal lawsuits
…Category: Regulatory
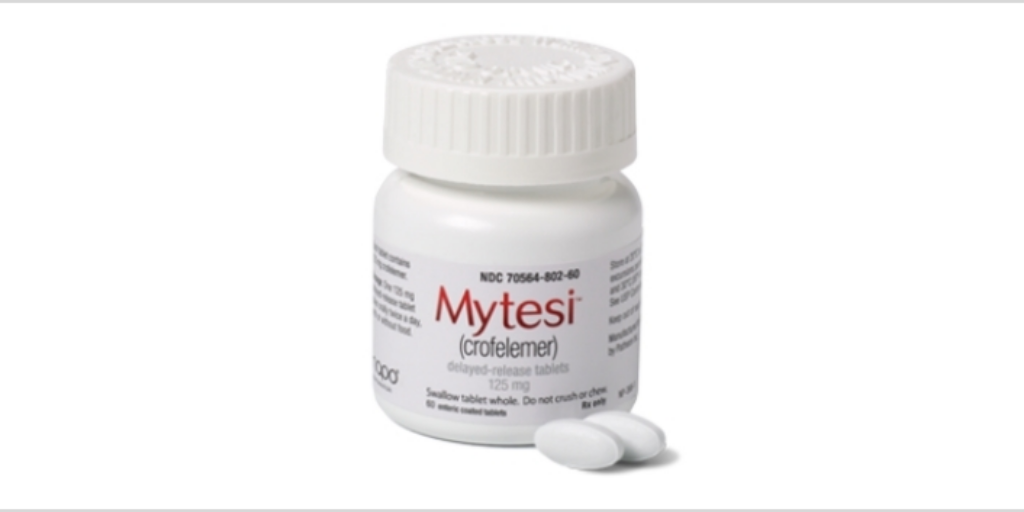
Napo Pharmaceuticals yesterday posted a public notice to 340B covered entities about how to obtain 340B pricing on Mytesi, an antidiarrheal for patients living with HIV/AIDS who take antiretroviral drugs.
Napo said in the notice on the U.S. Health
…U.S. Health and Human Services Secretary Xavier Becerra yesterday reiterated HHS’ “strong support for the 340B Drug Pricing Program” in a 29-page plan to lower drug prices.
Becerra’s Sept. 9 report to White House domestic and economic policy counselors
…With the deadline for hospitals to recertify their eligibility for 340B drug discounts arriving on Monday, it appears most facilities will remain qualified—at least for now.
Industry observers say the COVID-19 pandemic’s impact on hospitals’ eligibility for 340B could become
…Federal health officials have added New Jersey and New York to the list of states granted 340B enrollment flexibility due to Hurricane Ida.
The U.S. Health Resources and Services Administration (HRSA) posted the notice about the East Coast states’ addition
…The Arkansas Insurance Department is holding a public hearing this Friday morning on whether it should strike down as unconstitutional the nation’s first state law to address pharmaceutical manufacturer denials of 340B pricing on drugs when they are dispensed by
…Community health centers on Tuesday split their 340B dispute resolution petition against drug manufacturers Eli Lilly, Sanofi, and AstraZeneca into two—one against Lilly alone, the other against Sanofi and AstraZeneca together—to let federal officials commence proceedings against Sanofi and AstraZeneca.
…Medicare Part B reimbursement for physician-administered drugs is projected to rise from $18.7 billion in 2020 to $21.7 billion this year, and then to more than double to $46.2 billion by 2030, the Medicare Board of Trustees reported to Congress
…Louisiana and Mississippi health care providers eligible to participate in the 340B program may enroll immediately rather than having to wait until the next quarterly registration period Oct. 1 through 15, the U.S. Health Resources and Services Administration (HRSA) announced
…Michelle Herzog, long-time second-in-command at the U.S Health Resources and Services Administration’s Office of Pharmacy Affairs (OPA), has taken charge as OPA director on an acting basis.
A HRSA spokesperson confirmed yesterday morning that Herzog will oversee OPA temporarily now
…