Two major drug manufacturers are loosening their 340B contract pharmacy restrictions for states that have passed contract pharmacy access laws [...] …
Category: Regulatory
A federal judge today in Washington, D.C. scrutinized arguments from both manufacturers and the government in a closely watched hearing [...] …
A federal judge in Washington, D.C., is holding high-stakes hearings today on five drug industry lawsuits challenging the government’s opposition [...] …
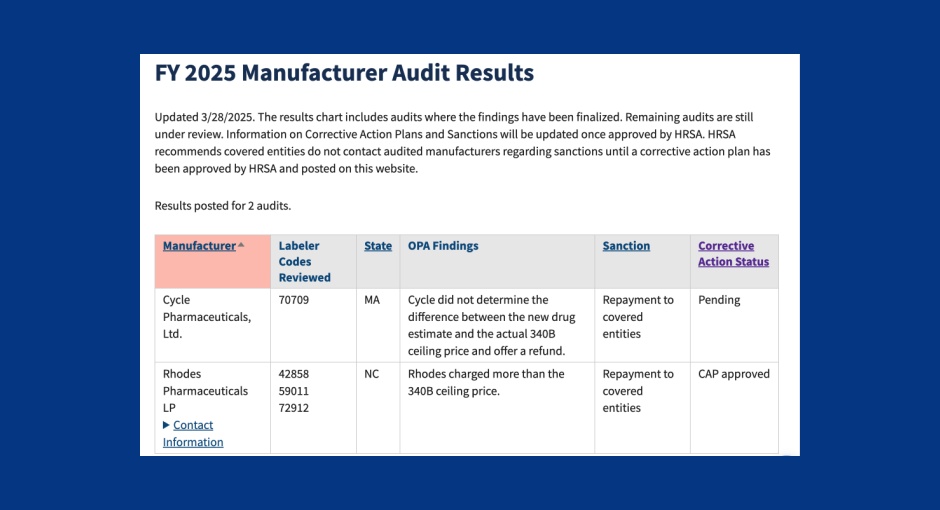
Recent Health Resources and Services Administration (HRSA) audits found that two drugmakers overcharged covered entities for their 340B drugs, fueling [...] …
A Japanese pharmaceutical giant and Massachusetts biotechnology company recently announced changes to their respective 340B contract pharmacy restrictions. Tokyo-based Takeda [...] …
The U.S. Department of Health and Human Services (HHS) is continuing its pushback against drugmaker attempts to unilaterally convert upfront [...] …
Minnesota covered entities will have additional 340B reporting requirements this year, but an extended deadline to submit that data, according [...] …
President Donald Trump’s (R) preliminary budget appears to call for “new authority to regulate all aspects of the 340B program,” [...] …
Hospital and health center leaders are pushing back against President Donald Trump’s (R) new executive order, criticizing its controversial 340B [...] …
News Alert
Trump Revives Controversial 340B Rule for Insulin, EpiPens in Sweeping Health Care Executive Order
President Donald Trump (R) last night issued a wide-ranging health care executive order that revives a controversial 340B policy from [...] …