A conservative think tank, which officials who served in President Donald Trump’s (R) first administration founded to advance his priorities, [...] …
Category: Regulatory
The Trump administration this week continued to oppose drug industry attempts to unilaterally convert 340B discounts to a rebate model, [...] …
In the Trump administration’s first legal filing on drugmaker 340B rebates, the U.S. Department of Health and Human Services (HHS) [...] …
Mehmet Oz, President Donald Trump’s (R) nominee to lead the Centers for Medicare & Medicaid Services (CMS), faced persistent questioning—primarily [...] …
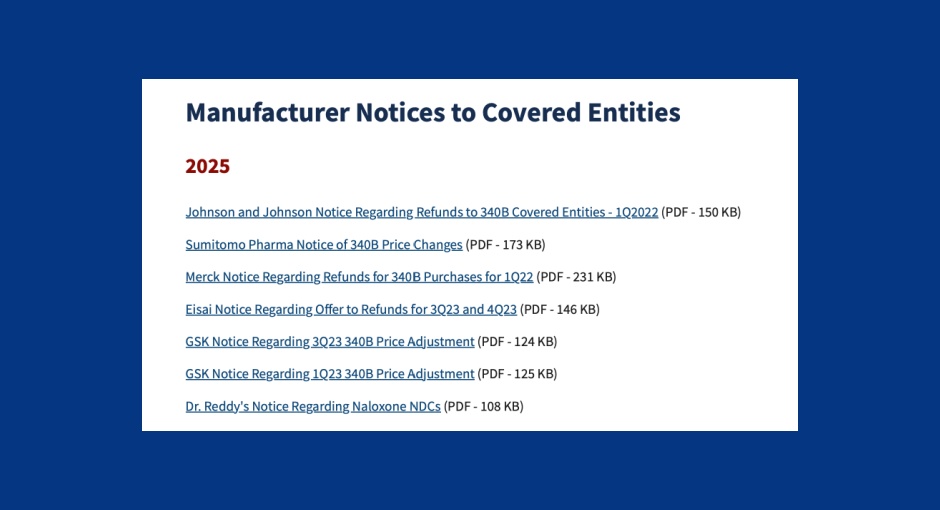
Two drugmakers recently notified covered entities of adjustments to their 340B drug ceiling prices and resulting refund eligibility. Sumitomo Pharma [...] …
Mehmet Oz, the celebrity heart surgeon and former U.S. Senate candidate that President Donald Trump nominated to lead the Centers [...] …
As state legislatures ramp up efforts to protect contract pharmacy access, drug industry lawsuits against existing state laws continue to [...] …
The University of Kansas Hospital (UKHA) this week sued the federal government for authorizing Johnson & Johnson (J&J) to audit [...] …
News Alert
Dark-Money 340B Attacks, Rebate Model Among Key Concerns at 340B Coalition Winter Conference
Concerns over a highly charged and misleading dark-money campaign targeting state contract pharmacy access bills and the legal fight surrounding [...] …
Sagebrush Health, a Nevada-based STD clinic, has voluntarily dismissed its 340B lawsuit against the federal government—a move the clinic says [...] …