A controversial GOP-backed plan to overhaul the 340B program continued to draw pushback from provider advocates—as well as muted or [...] …
Category: Pharma Industry
Four major hospitals recently filed an emergency appeal to block Johnson & Johnson (J&J) from auditing their 340B programs—three months [...] …
A U.K.-based drugmaker recently announced it will soon exempt covered entities in two additional states from its 340B contract pharmacy [...] …
Breaking News
Federal Appeals Court Unanimously Upholds Mississippi’s 340B Contract Pharmacy Access Law Against AbbVie Challenge
A federal appeals court today unanimously upheld Mississippi’s 340B contract pharmacy access law, rejecting AbbVie’s bid to block enforcement of [...] …
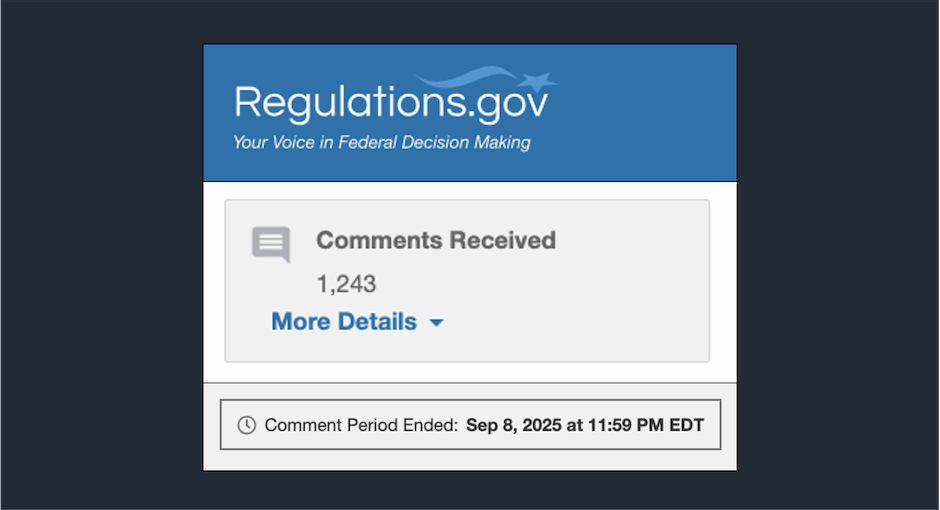
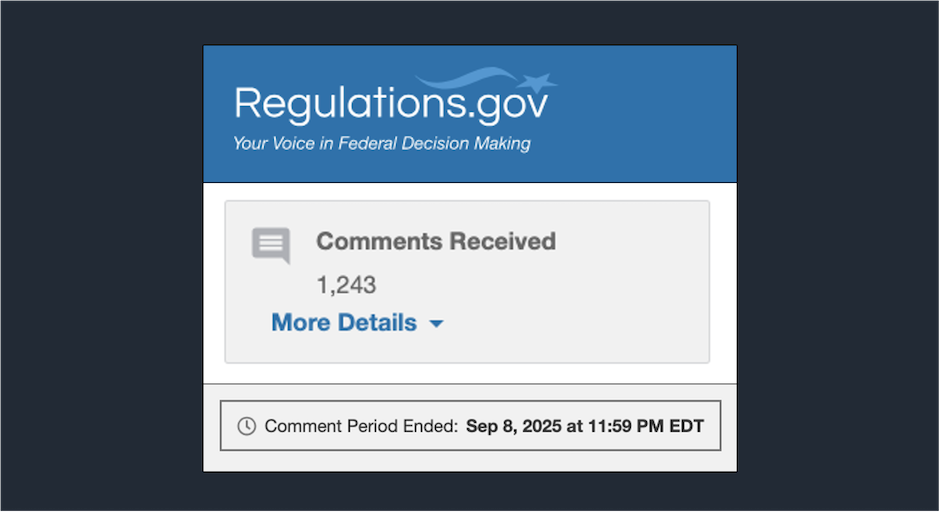
The Health Resources and Services Administration (HRSA) has now published 1,116 of the 1,243 comments it received on its controversial [...] …
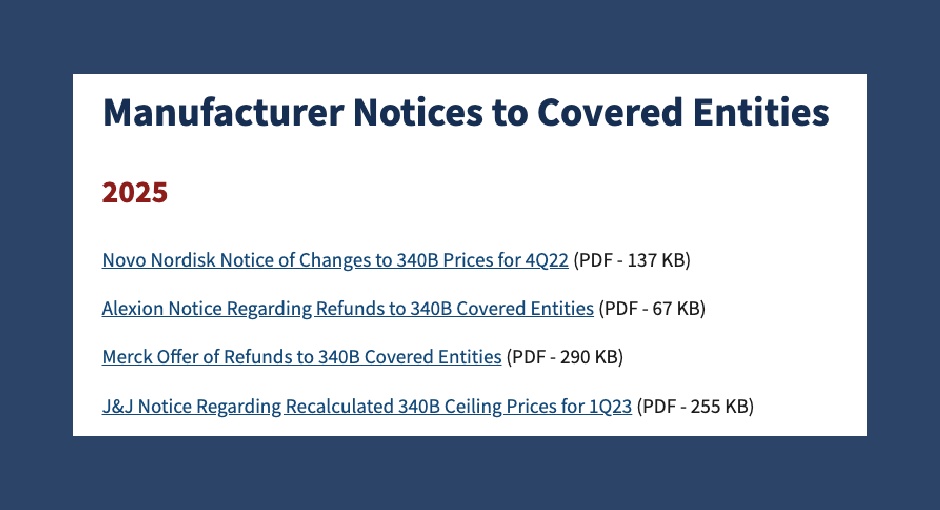
Four major drugmakers recently announced refunds to 340B covered entities that purchased certain medications between 2022 and 2024, following recalculations [...] …
Breaking News
Carter, Harshbarger Reintroduce Controversial 340B ACCESS Act in U.S. House—This Time Without a Key Supporter
Two key U.S. House Republicans and vocal 340B program critics on Wednesday reintroduced controversial 340B overhaul legislation—but unlike last year’s [...] …
A tough-questioning federal appeals court panel yesterday held back-to-back oral arguments on drug industry legal challenges to Maryland and West [...] …
The Health Resources and Services Administration (HRSA) received more than 1,200 comments on its controversial 340B rebate pilot program ahead [...] …
North Dakota officials have transferred a drug industry lawsuit over the state’s new 340B contract pharmacy access law from state [...] …