Drugmakers GlaxoSmithKline and Vertex Pharmaceuticals will offer refunds to 340B providers related to the purchases of several of their high-earning [...] …
Category: Regulatory
The AIDS Healthcare Foundation (AHF) recently urged a federal appeals court to reverse a lower court’s decision to dismiss its [...] …
New Medicaid Policy Change Could Reduce 340B Savings, Cause Drug Discontinuations, Warn Stakeholders
A Medicaid policy change that took effect at the start of 2024 could indirectly lead to lower 340B savings and [...] …
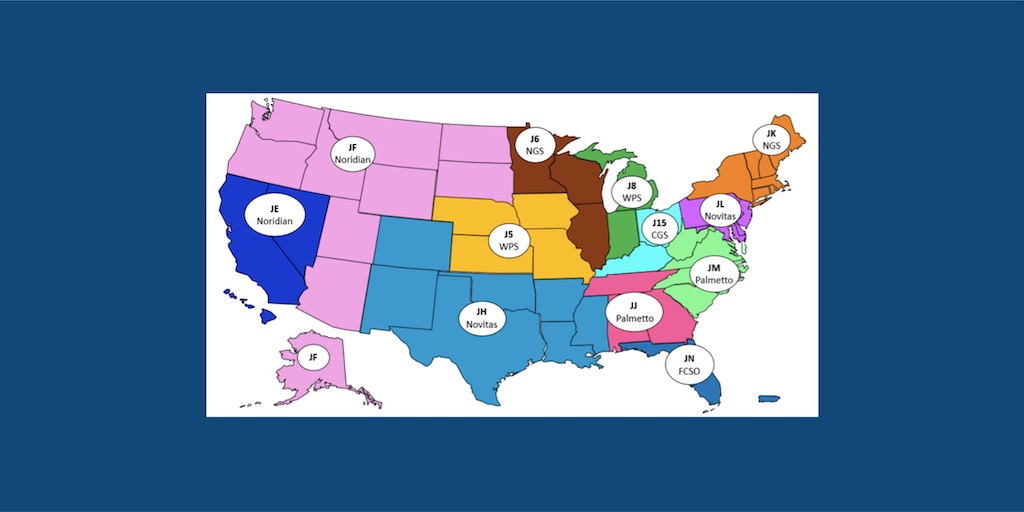
Medicare administrative contractors (MACs) covering more than half the states have initiated lump sum repayments to 340B hospitals, after getting [...] …
Drugmakers issued a record 41 refunds to 340B covered entities in 2023. And Merck is the first to announce it [...] …
The Health Resources and Services Administration (HRSA) has announced plans to host a webinar for federal grantees just days prior [...] …
The nation’s leading hospital group warned the decision by federal regulators not to address 340B policy in a new proposed [...] …
Federal regulators have approved a first-time 340B provider audit by a drug manufacturer to examine processes to prevent duplicate discounts [...] …
Federal regulators recently deferred a decision on whether to add identifiers to 340B drugs purchased under Medicare Part D. The [...] …
In the wake of a high-profile federal court ruling casting doubt on the definition of eligible “patient” in the 340B [...] …