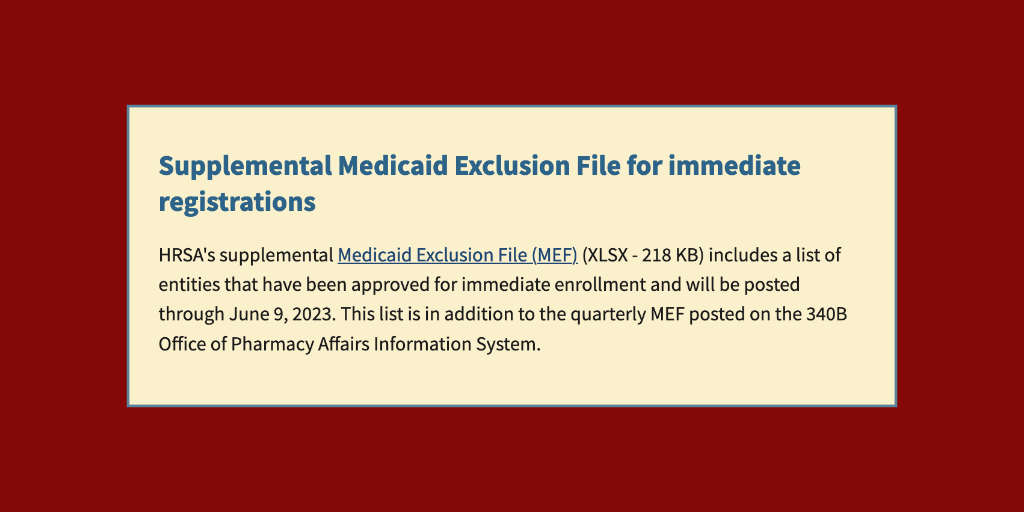
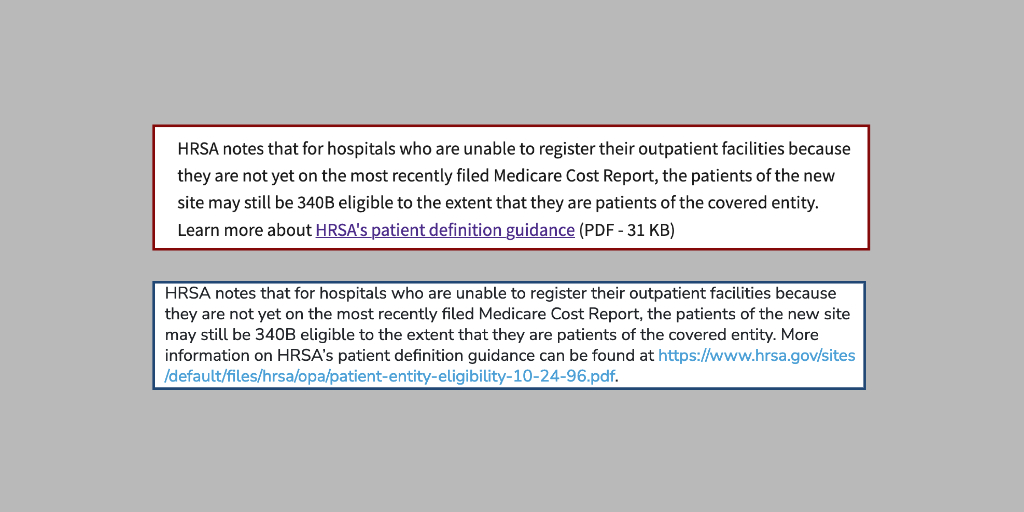
Health care providers in Guam eligible to participate in the 340B program may do so immediately, rather than having to wait for the next normal quarterly registration period July 1-15, the U.S. Health Resources and Services Administration said yesterday.
Secretary
…