SPONSORED CONTENT
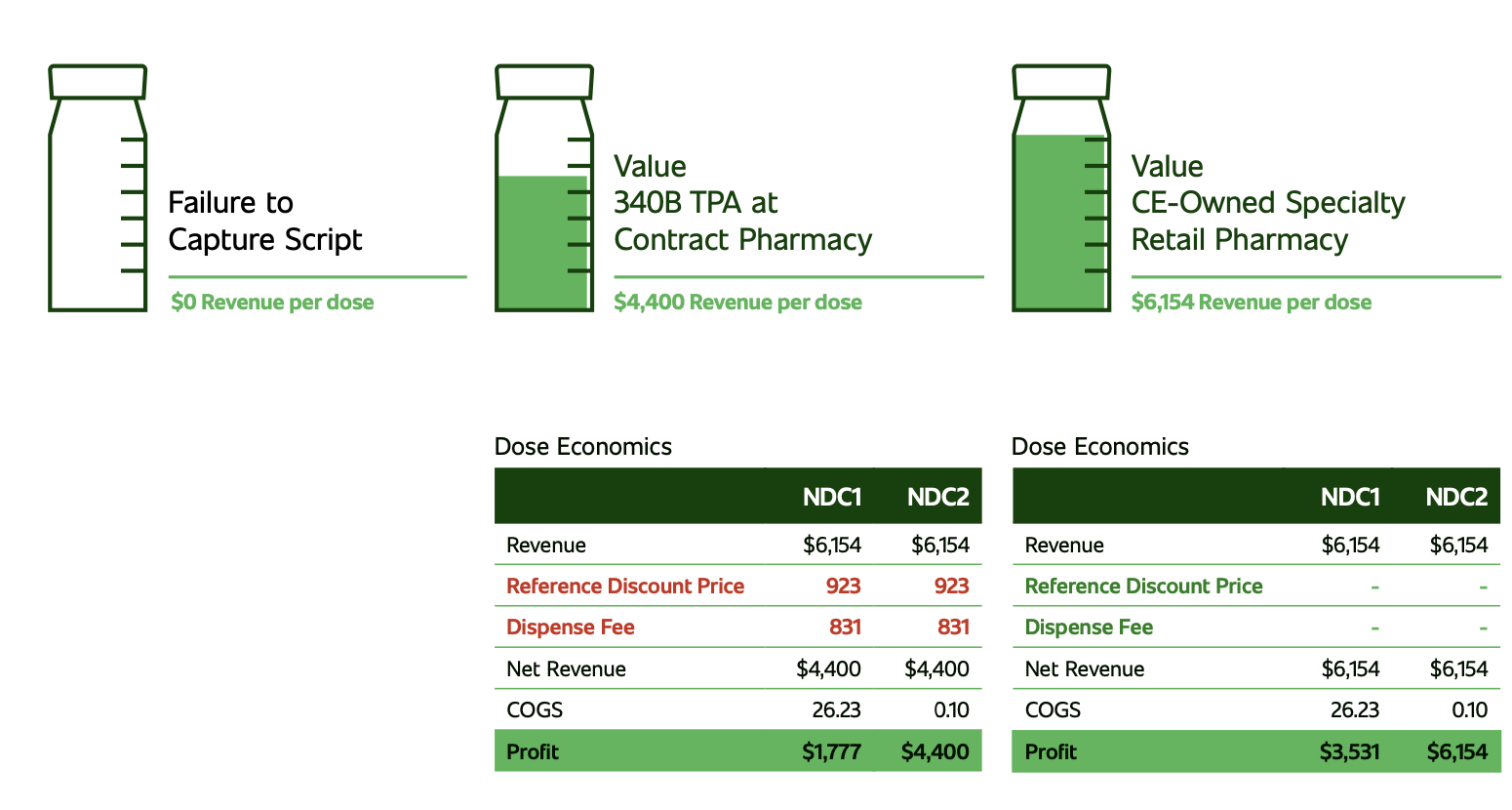
For 340B program directors, complying with the U.S. Health Resources & Services Administration’s (HRSA’s) requirements is top of mind, all the time. That’s partly because maintaining their employer’s 340B program directly ties to profitability and sometimes survival. Covered entities need 340B revenue to meet the program’s purpose “to stretch scare resources as far possible, reach more eligible patients and provide more comprehensive services.” Complying with the program requires a constant focus on guidelines and regulations.
Along with protecting program benefits and monitoring changing requirements, 340B program managers also hear politicians, pharmaceutical manufacturers and the press call for more oversight by the federal government. Manufacturers are concerned about program growth. According to HRSA, discounted purchases under the 340B Program hit $44 billion in 2021, a 16-percent uptick from 2020. The volume may have slowed down with the recent manufacturer restrictions on use of 340B discounts in the contract pharmacy setting but
…