The Biden administration proposes once again to push back, this time from March 22 to July 20, the effective date of the Trump administration’s controversial final rule to require health centers to provide insulin and injectable epinephrine to low-income patients
…Category: Federal
Drug manufacturer Sanofi on Monday struck back at the U.S. Justice Department (DOJ) for opposing its Feb. 2 motion in a federal lawsuit requesting an injunction against the 340B program’s new administrative dispute resolution (ADR) process.
“The government is eager
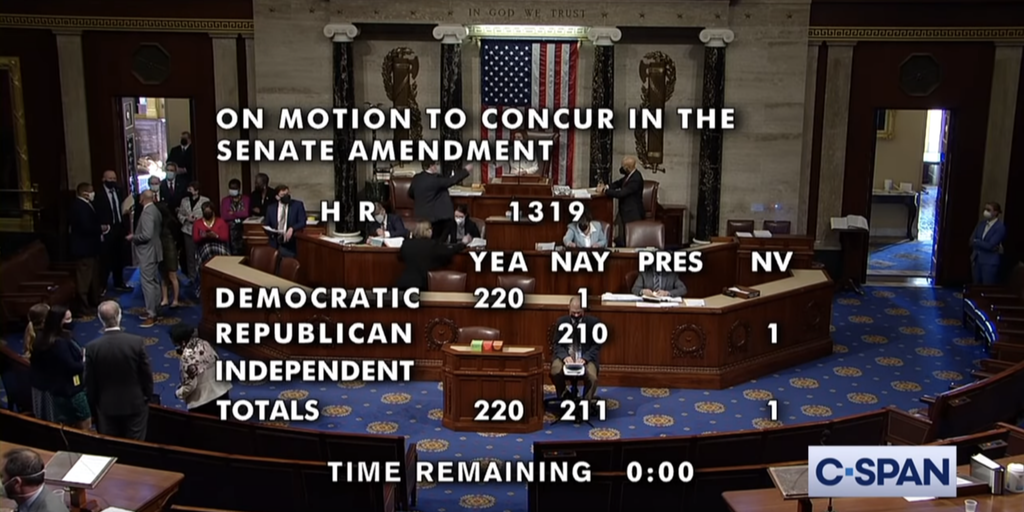
…The U.S. House on Wednesday passed and sent to President Biden a $1.9 trillion COVID-19 relief and economic recovery bill with provisions affecting the 340B program and its participants. Biden will sign the bill at the White House tomorrow.
The
…The U.S. Senate voted 50 to 49 along party lines early Saturday morning to approve an amended version of the House’s $1.9 trillion COVID-19 relief bill. According to House Majority Leader Steny Hoyer’s (D-Md.) office, the House could vote on
…Hospital groups that recently filed a motion to intervene in opposition to drug manufacturer Eli Lilly’s lawsuit against the federal government over 340B contract pharmacy requirements also are trying to intervene in similar suits against the government by manufacturers AstraZeneca,
…The U.S. Senate Finance Committee yesterday split 14-14 along party lines on whether to favorably report California Attorney General Xavier Becerra’s nomination to serve as U.S. Health and Human Services (HHS) secretary to the full Senate.
There is a 50-50
…More than 70 percent of U.S. spending on top-selling “partial orphan drugs”—drugs for rare diseases that also are approved to treat common diseases—is for non-orphan indications, a study in latest edition of Health Affairs concludes.
Makers of partial orphan drugs
…In a concession to drug companies, Senate Democrats reportedly have agreed to delay by one year—from Jan. 1, 2023 to Jan. 1, 2024—the effective date of language in the $1.9 trillion COVID-19 relief bill that might lead to 340B ceiling
…The U.S. Justice Department (DOJ) has slammed a second drug manufacturer’s motion before a federal court to block implementation of the 340B program’s new administrative dispute resolution (ADR) process.
First, on Feb. 16 in a federal district court in
…