UPDATED Thursday, Feb. 17, 2022, 4:00 p.m. EDT—The U.S. Health Resources and Services Administration (HRSA) said this afternoon, “The 340B program is an essential component of the safety net system that helps make health care and prescription drugs more affordable for millions of Americans. HRSA respectfully disagrees with the recent District Court opinion, and we will continue to work to ensure the program delivers for underserved patient populations and frontline health care providers.”
Yesterday’s court decision in AstraZeneca’s 340B contract pharmacy lawsuit made the score 6-0 in favor of drug manufacturers in their fight with the federal government over the legality of its May 17, 2021, letters telling Astra, Lilly, Novartis, Novo Nordisk, Sanofi, and United Therapeutics that their actions have resulted in overcharges and are in direct violation of the 340B statute.
Four judges—covering a total of six lawsuits—were asked to rule on the letters’ legality. All found reasons to set aside and vacate the letters. As of this morning, all six letters are dead.
One of the four judges has been asked to rule on the legality of a seventh letter, to Boehringer Ingelheim. That case is on hold.
AstraZeneca has not responded yet to a request for comment. Hospital group 340B Health last night called on the government to appeal.
Judge Stark’s Ruling
U.S. District Judge Leonard Stark of the District of Delaware was the last of the four judges to rule. He issued his decision early yesterday evening.
Stark said the U.S. Health Resources and Services Administration (HRSA) did not comply with the federal Administrative Procedure Act when it issued its 340B program violation letter to Astra.
In July 2021, Stark vacated and set aside the U.S. Health and Human Services (HHS) General Counsel’s December 2020 advisory opinion that the 340B statute compels drug manufacturers to offer 340B pricing when covered entities use contract pharmacies.
In his ruling yesterday, Stark said HRSA’s May 2021 violation letter to AstraZeneca is based on essentially the same flawed interpretation of the 340B statute as the advisory opinion that he vacated.
“Because the violation letter advances essentially the same statutory interpretation as the one contained in the opinion, the court’s previous analysis of the 340B statute applies here with equal force,” Stark wrote.
Stark said he previously found that the text of 340B statute never mentions pharmacies, which he said strongly indicates “that the statute does not compel any particular outcome with respect to covered entities’ use of pharmacies.” The omission is notable because the statute “explicitly refers to certain affiliates of covered entities,” Stark continued. It is hard to imagine that Congress precisely enumerated 15 different types of covered entities “and then intended to impliedly sweep in sales implicating contract pharmacies,” he said.
Stark also said 340B’s legislative history cuts against the government’s position. In 1996, he said, “Congress considered but ultimately rejected language referring to drugs ‘purchased and dispensed by, or under a contract entered into for on-site pharmacy services with’ covered entities.”
“The exclusion of that language indicates that Congress did not clearly intend for drug manufacturers to be required to facilitate sales of covered drugs for dispensing by an unlimited number of contract pharmacies,” Stark wrote.
Stark also struck down and set aside HRSA’s violation letter to Astra because, he said, HRSA’s position about whether manufacturers must provide 340B pricing regardless of the dispensing mechanism has shifted over time.
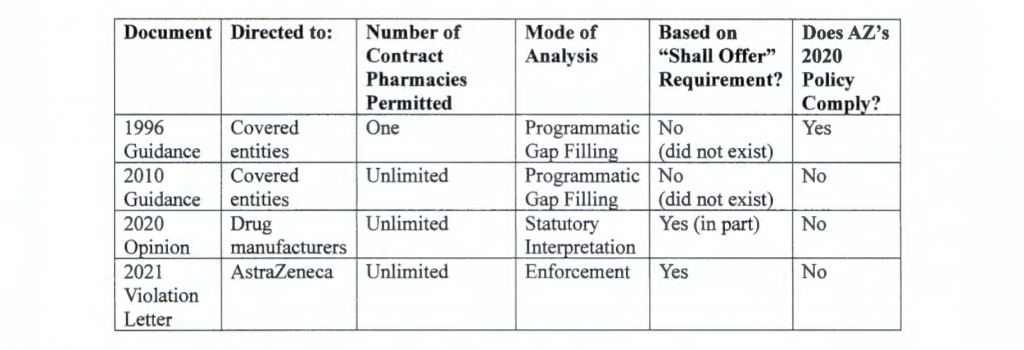
Stark included a table in his opinion summarizing differences among HRSA’s 1996 and 2010 contract pharmacy guidance, the 2020 HHS advisory opinion, and the 2021 violation letter. He said it was notable that “AstraZeneca’s new policy regarding 340B drugs would have complied with the parameters set out in the 1996 guidance, while the violation letter determines that AstraZeneca’s new policy does not comport with the agency’s current understanding of the 340B statute.”
Stark also faulted the government for belatedly in the lawsuit citing HRSA’s 1994 340B program guidance, in which HRSA rejected a commenter’s recommendation that manufacturers not be required to sell directly to a contract pharmacy. HRSA noted in its rejection that covered entities often use contract pharmacies.
Before citing its 1994 guidance, HRSA consistently said that its 1996 guidance was its first guidance document relevant to the case, Stark said.
Also, he said, on the one hand the 1994 guidance “suggests that drug manufacturers should be required to facilitate sales of 340B drugs dispensed by contract pharmacies.” But on the other hand, he said, HRSA made clear that the guidance document was directed to entities, not manufacturers, and “was never intended to impose any duties on drug manufacturers.”
“The 1994 guidance is even more confusing when considered alongside the 1996 and 2010 guidance documents,” Stark said. “If the 1994 guidance is read as having approved arrangements involving multiple contract pharmacies, then the government would have to explain how and why it took a narrower view in the 1996 guidance, when it limited covered entities to using only a single contract pharmacy. Later, in 2010, it similarly would have to explain how and why it was returning to a broader view. The administrative record does not reveal any credible explanation, and the government has not offered one in the arguments before this court.”
How the Other Courts Ruled
Groups representing 340B covered entities have emphasized what, for entities, are the bright sides of the other court rulings decisions about the legality of HRSA’s violation letters to the six manufacturers.
They note that in Lilly, Novo Nordisk, and Sanofi’s cases (the latter two were decided together), judges said that the companies had no right to impose unilateral, extra-statutory conditions on 340B pricing, and that the government has authority to enforce the 340B statute.
Still, both judges erased HRSA letters telling Lilly, Novo Nordisk, and Sanofi that they broke the law.
In Lilly’s case, U.S. Senior District Judge Sarah Evans Barker ruled that HRSA’s violation letter was arbitrary and capricious in violation of the Administrative Procedure Act (APA). Barker said she voided HRSA’s findings against Lilly because HRSA has espoused the conflicting views that (1) manufacturers must comply with 340B statutory requirements regardless of how entities dispense drugs, (2) HRSA lacks authority to issue binding contract pharmacy regulations and operates instead with guidance that limits its enforcement authority, and (3) it is up to the 340B administrative dispute resolution (ADR) process to determine whether Lilly’s policies are legal or illegal.
In Novo Nordisk, and Sanofi’s cases, U.S. Chief District Judge Freda Wolfson of the District of New Jersey struck down and vacated HRSA’s violation letters for a different reason. She said she did so “to the extent that such determinations may depend on the number of permissible contract pharmacy arrangements under the 340B statute.” The government, she said, must “undertake a more complete assessment of the status quo as to contract pharmacy arrangements to determine whether it is permissible under the 340B statute to enforce a one-size-fits-all contract pharmacy policy, or whether more specific and holistic guidance is necessary.”
U.S. District Judge Dabney Friedrich’s ruling in Novartis and United Therapeutics’ cases was, until yesterday, the least favorable to covered entities. In striking down HRSA’s letters, she rejected the government’s position that the 340B statute’s plain meaning, purpose, and structure preclude manufacturers from imposing “any” conditions on 340B pricing. However, she did rule that manufacturers can’t impose “all” conditions.
Stark’s ruling yesterday is arguably the least favorable of them all to the covered entity position on manufacturers’ responsibility to honor 340B contract pharmacy arrangements.


