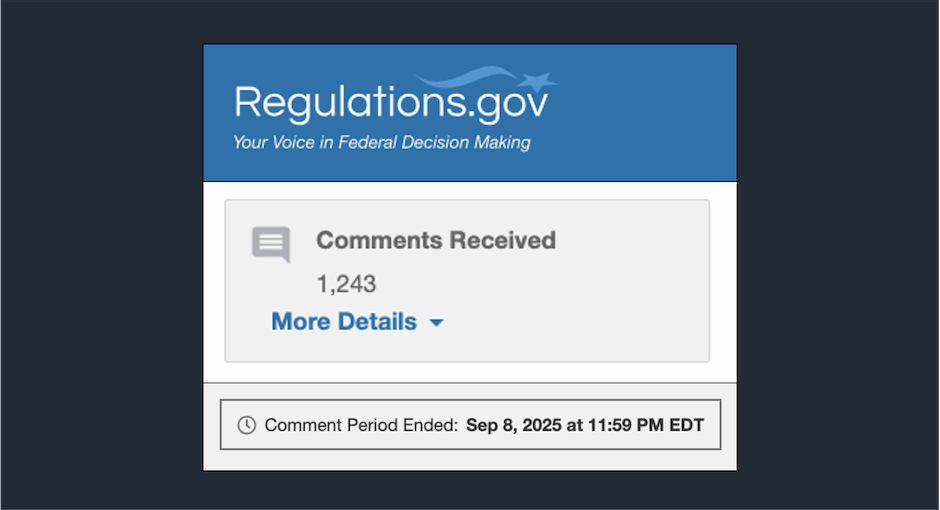
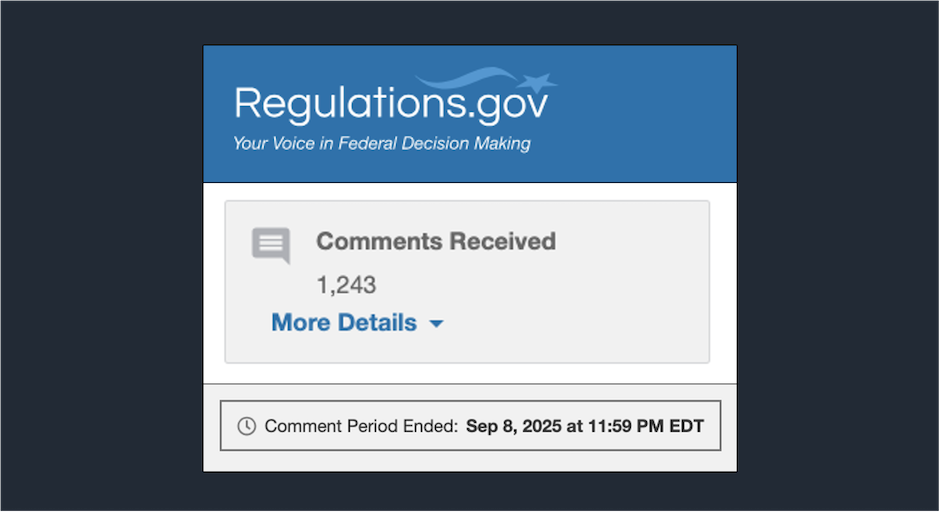
The Health Resources and Services Administration (HRSA) has now published 1,116 of the 1,243 comments it received on its controversial [...] …
Category: Federal
A federal appeals court recently heard arguments in a case, which a group of Texas hospitals filed challenging a Department [...] …
Four major drugmakers recently announced refunds to 340B covered entities that purchased certain medications between 2022 and 2024, following recalculations [...] …
Breaking News
Carter, Harshbarger Reintroduce Controversial 340B ACCESS Act in U.S. House—This Time Without a Key Supporter
Two key U.S. House Republicans and vocal 340B program critics on Wednesday reintroduced controversial 340B overhaul legislation—but unlike last year’s [...] …
The Health Resources and Services Administration (HRSA) received more than 1,200 comments on its controversial 340B rebate pilot program ahead [...] …
A bipartisan group of 163 U.S. House members yesterday sent Health and Human Services (HHS) Secretary Robert F. Kennedy, Jr. [...] …
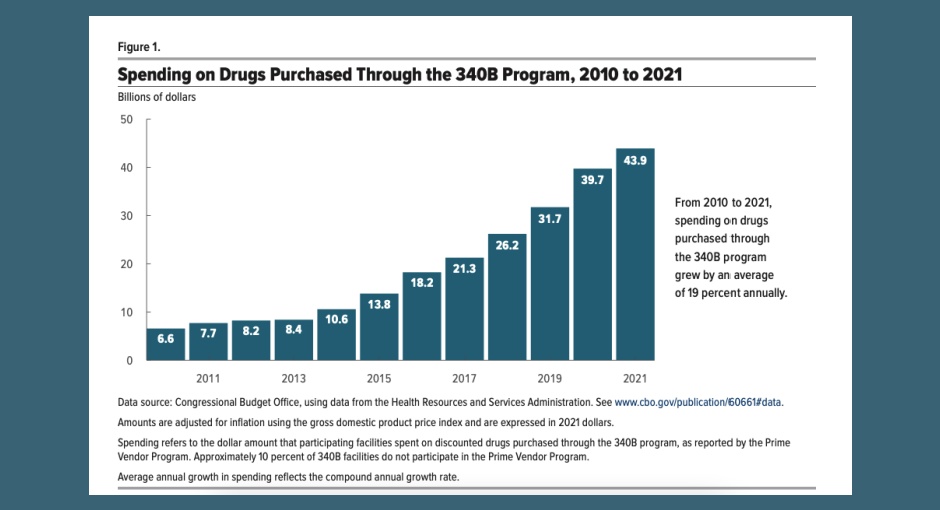
340B covered entities in the prime vendor program (PVP) spent nearly $44 billion on 340B drugs in 2021—up from $6.6 [...] …
A national hospital trade group urged federal officials Monday to investigate whether five major drugmakers engaged in anticompetitive practices while [...] …
A South Carolina Republican U.S. House lawmaker recently honored the life and legacy of Sue Veer, a nationally recognized leader [...] …
Editor’s Note: With 340B-related litigation heating up in several states following the enactment of new contract pharmacy access laws, 340B [...] …