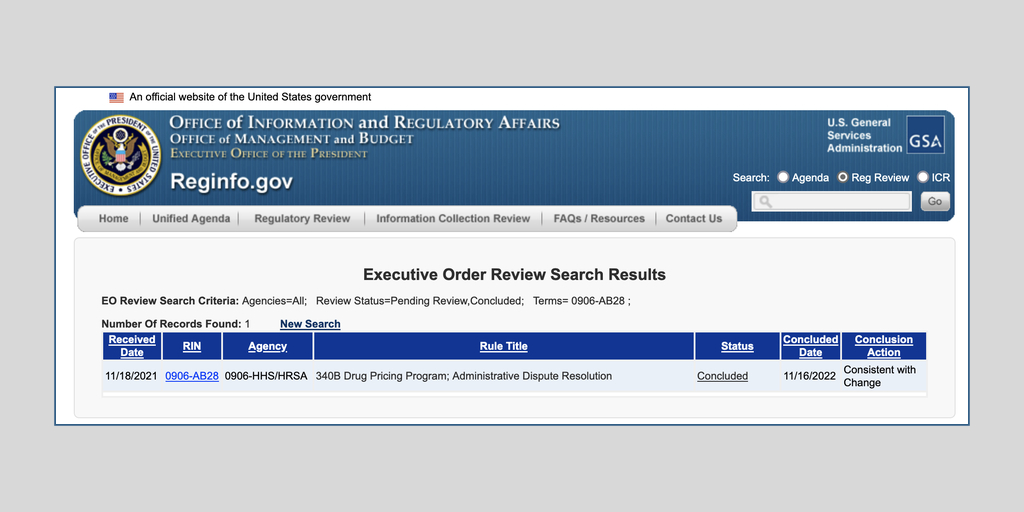
The U.S. Health Resources and Services Administration this morning proposed a replacement for its two-year-old 340B administrative dispute resolution process. Comments are due Jan. 30.
“HRSA has encountered policy and operational challenges with implementation of the
2020 final rule,” the
…