A leading not-for-profit HIV and AIDS treatment organization amplified its 340B advocacy recently with an op-ed in a Washington, D.C., [...] …
Category: Federal
Breaking News
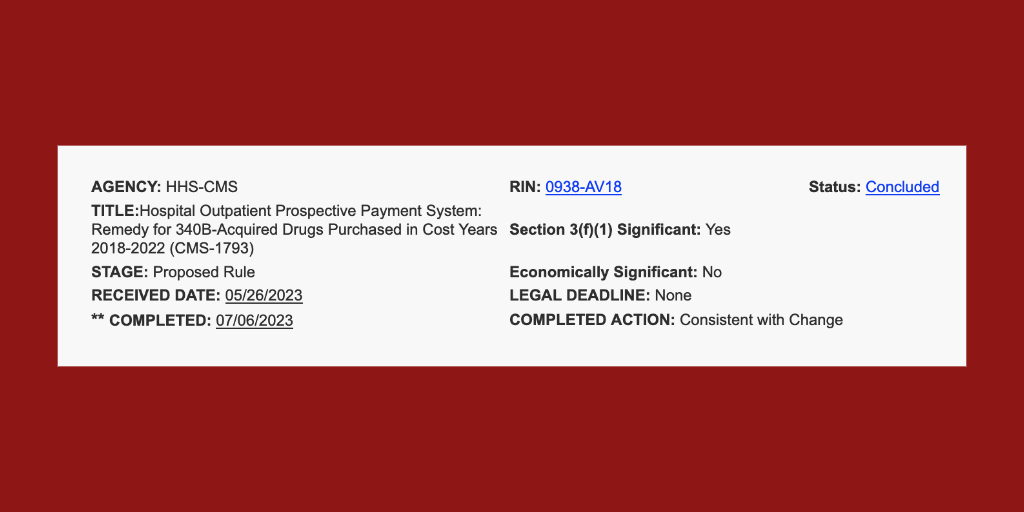
CMS Proposes $9 Billion in Lump Sum Payments to Reimburse Hospitals for Past 340B Part B Drug Payment Cuts
The U.S. Centers for Medicare & Medicaid Services plans to provide lump sum Medicare Part B drug payments to 340B [...] …
The White House yesterday gave the U.S. Centers for Medicare & Medicaid Services the go-ahead to publish its proposed remedy [...] …
Apexus, the federally contracted 340B prime vendor, and AIDS Healthcare Foundation are meeting with a mediator to try to settle [...] …
The latest guidance to implement first-time drug price negotiation under Medicare said federal officials still are looking for a process [...] …
U.S. Sen Bill Cassidy (La.), the top Republican on the Senate Health, Education, Labor, and Pensions Committee, said in an [...] …
The national health center group promoting 340C—a proposed alternative to the 340B drug pricing program for health centers, federal grantees, [...] …
The National Rural Health Association has intensified its 340B advocacy work with a new congressional campaign and set of policy [...] …
A bill amending the 340B statute to explicitly require drug makers to unconditionally sell and deliver 340B drugs dispensed by contract pharmacies is expected to be introduced just before or during the 340B Coalition summer conference July 10-12 near Washington,
…Two hospital groups separately urged Biden administration officials this month to not divert funding from non-340B hospitals and some 340B hospitals in order to repay other 340B hospitals for almost five years’ worth of illegal Medicare Part B drug payment
…