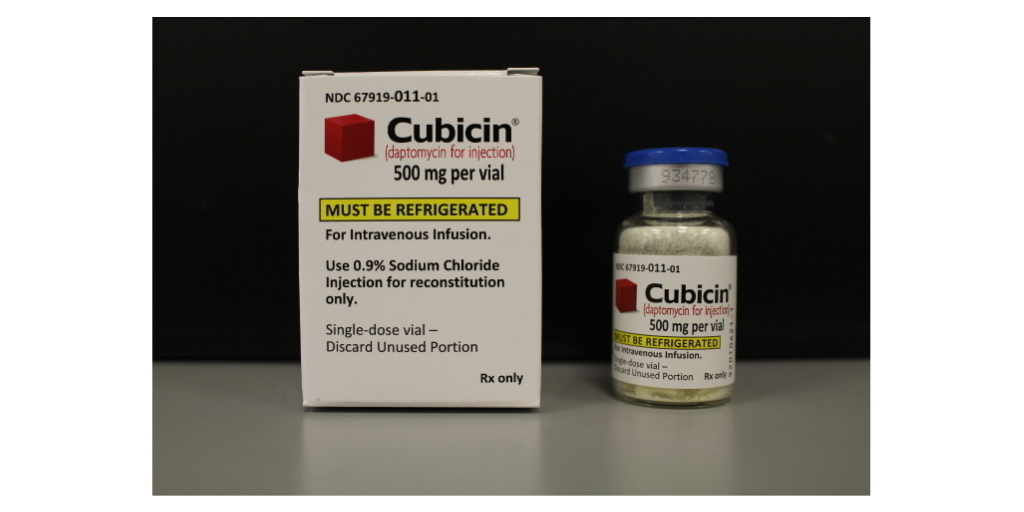
Drug manufacturer Merck is paying refunds for overcharges on 340B drugs purchased during the first two quarters of 2020.
Merck recently asked the U.S. Health Resources and Services Administration to post notices about its 340B ceiling price recalculations on the
…