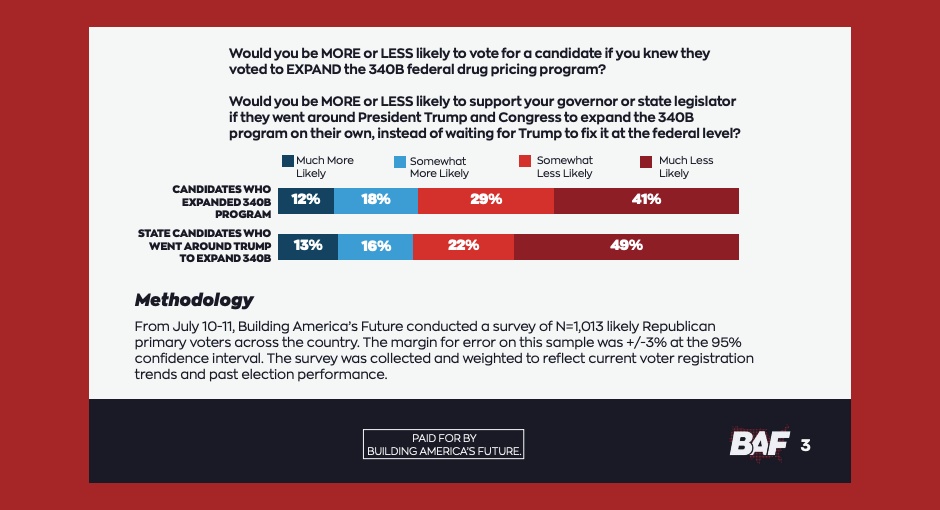
A majority of 340B hospitals surveyed said the costs associated with implementing drugmakers’ proposed rebate models would hinder their ability [...] …
Category: Federal
The dark money group behind a campaign targeting GOP-led states’ recent efforts to enact 340B contract pharmacy access laws released [...] …
Three patient groups are urging a federal appeals court to back drugmaker 340B rebate models they argue would bring transparency [...] …
Two major drugmakers recently announced new states that will be exempt from their 340B contract pharmacy restrictions following their enactment [...] …
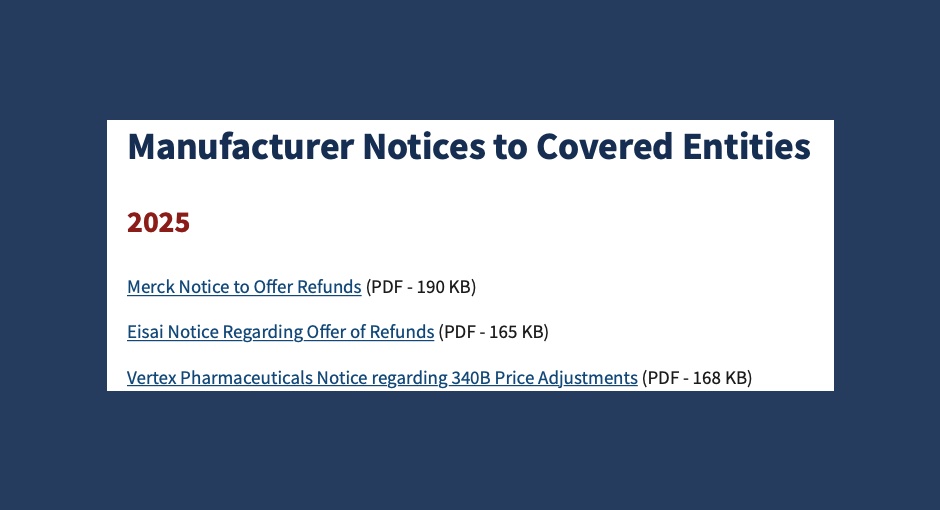
Three drugmakers recently announced refunds to 340B covered entities that purchased certain medications between 2022 and 2025 following recalculations of [...] …
Nearly 80 advocates marched toward Capitol Hill yesterday in a record turnout for the fourth annual “Defend 340B March” in [...] …
The debate over federal drug pricing reform could garner newfound attention on Capitol Hill now that Congress has wrapped up [...] …
Although President Donald Trump’s nearly 900-page “big beautiful bill” never mentions the 340B program, experts warn that the new law’s [...] …
News Alert
Matsui, Welch Reintroduce 340B PATIENTS Act in Congress During 340B Coalition Summer Conference
The two congressional Democrats behind the 340B PATIENTS Act—a 2024 federal bill that would’ve codified providers’ ability to use 340B [...] …
A key federal regulator highlighted the Health Resources and Services Administration’s (HRSA) legal fight against proposed drugmaker 340B rebate models [...] …