Federal health officials last week defended their plan to wait until next year to say how they will reimburse 340B hospitals for illegal Medicare Part B drug payment cuts in place for the past five years after hospital groups told
…Category: Federal
The U.S. Health Resources and Services Administration has ordered Pennsylvania-based generic drug manufacturer KVK Tech to repay 340B covered entities for program violations found during an audit.
HRSA posted its audit findings Nov. 15. “KVK-Tech failed to refund covered entities for
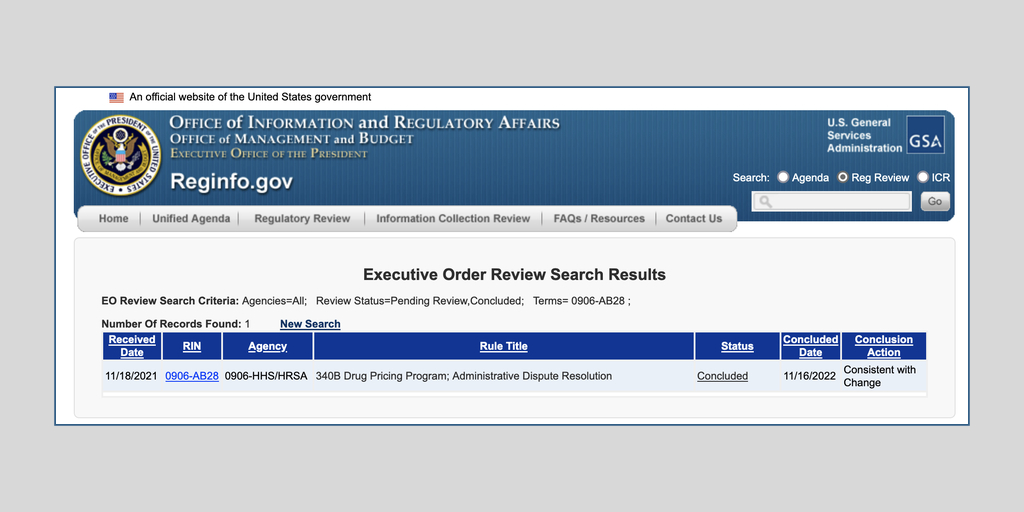
…The White House yesterday unexpectedly gave the U.S. Health Resources and Services Administration permission to publish a proposed replacement for HRSA’s December 2020 340B administrative dispute resolution final rule.
The Office of Management and Budget announced on its website Nov.
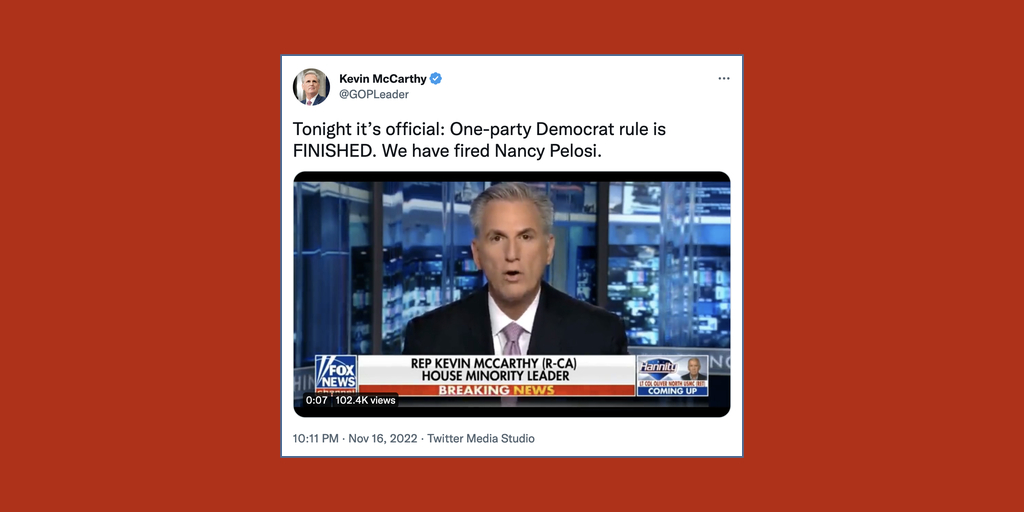
…The Republican Party won control of the U.S. House last evening, securing the requisite 218th seat for majority status after the Associated Press declared Rep. Mike Garcia (R) the winner of a race in California.
Democrats on Nov. 12 won
…Two judges on a three-judge federal appeals court panel this morning challenged a federal government lawyer’s stance that federal law forbids drug manufacturers from limiting how their drugs may be distributed when they offer to sell the drugs at reduced
…A federal appeals court in Philadelphia this morning is hearing arguments in AstraZeneca, Novo Nordisk, and Sanofi’s lawsuits challenging federal agency findings that the drug companies’ 340B contract pharmacy policies are illegal and must stop or the firms could be
…Democrats will still control the U.S. Senate for the next two years, it became clear over the weekend, but control of the House has not yet been decided one week after the Nov. 8 midterm elections.
As of early this
…The 340B statute should be amended to extend discounts to emergency-use drugs to treat COVID-19 as a first step in rebuilding the COVID-19 safety net, an opinion piece published yesterday in the medical journal Health Affairs says.
…Partisan control of Congress for the final two years of President Biden’s term in office was still undecided this morning, two days after Tuesday’s midterm elections.
Major national news organizations say Republicans are favored to take control of the House,
…