Now that a federal appeals court in Washington, D.C., has heard arguments in Novartis and United Therapeutics’ 340B contract pharmacy lawsuits, the scene shifts to Chicago, where an appeals court will hear arguments Monday morning in Eli Lilly’s companion case.
…Category: Federal
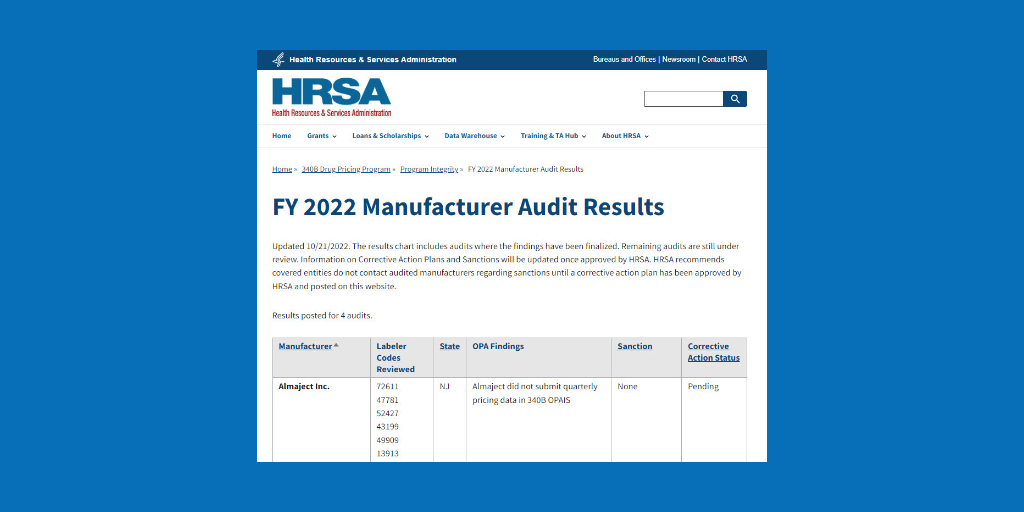
The U.S. Health Resources and Services Administration (HRSA) has cited Almaject, a New Jersey-based maker of generic injectables, with failing to submit quarterly pricing data into the 340B Office of Pharmacy Affairs Information System, although the agency did not impose
…Two of three judges on a federal appeals court panel yesterday repeatedly asked lawyers during a highly anticipated hearing in Washington, D.C., what standard the court should use to decide whether drug manufacturers’ 340B contract pharmacy conditions are legal.
The
…Congress’ nonpartisan research office has issued a four-page backgrounder on litigation over the 340B contract pharmacy program and a two-pager on the entire drug discount program. They could be a sign that 340B hearings and/or bills are coming.
The Congressional
…The federal government and a Tennessee health system are meeting with a mediator in connection with a whistleblower lawsuit that alleges that the system paid Memphis-area cancer physicians for referrals that generated $50 million in profits in one year alone
…Breaking News
In Key Hearing, Appeals Court Panel Seems to Lean in Favor of Some Drug Manufacturer Conditions on 340B Pricing
Two of three judges on a federal appeals court panel strongly indicated this morning that they reject the federal government’s position that nothing in the 340B statute gives drug manufacturers the right to put any conditions on offers of 340B
…The U.S. Health Resources and Services Administration yesterday referred drug manufacturer Merck to the Department of Health and Human Services Office of Inspector General for possible imposition of civil fines over Merck’s 340B contract pharmacy restrictions.
Public Health Services Lt.
…The U.S. Centers for Medicare & Medicaid Services is making computer file changes to restore hospitals’ Part B payments for 340B-purchased drugs to average sales price plus 6% for the rest of 2022 after paying them almost 30% less since
…The U.S. Department of Health and Human Services (HHS) late last week extended the COVID-19 public health emergency (PHE) to mid-January, giving some 340B-eligible entities more time for immediate enrollment in the program without having to wait for the next
…The National Association of Community Health Centers is asking to meet with U.S. Health and Human Services Secretary Xavier Becerra to discuss drug companies’ “unlawful restrictions” on 340B drug shipments to contract pharmacies.
NACHC told Becerra in an Oct. 14
…