The congressman who represents Richmond, Va., told a health system Wednesday he rejects its reasons for ceasing intensive care, maternity, and other vital services at a Richmond hospital in a mainly Black neighborhood despite the hospital allegedly earning millions of
…Category: Federal
President Biden will sign an executive order today directing the U.S. Health and Human Services Department (HHS) “to consider additional actions to further drive down prescription drug costs,” the White House said this morning.
“Under the Executive Order, HHS will
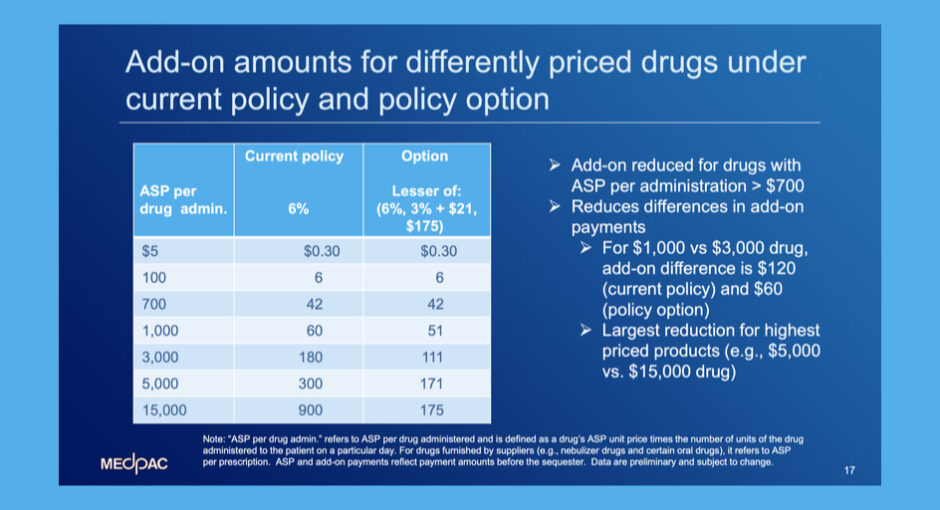
…The American Hospital Association is urging the Medicare Payment Advisory Commission to abandon a Medicare Part B drug reimbursement policy option it is considering making to Congress, arguing that the proposed change would hurt 340B hospitals’ ability to provide care
…The 340B program “has always been uniquely federal in nature” and “there is no room for states, pharmacies, or other interests to step in and try to supplant” the federal government’s exclusive control over it, the brand drug industry said
…A federal district judge on Monday cleared the way for the Centers for Medicare and Medicaid Services (CMS) in as quickly as two weeks to resume paying hospitals at the rate of average sales price (ASP) plus 6% for their
…Five Washington insiders steeped in knowledge about the 340B program will take part in a 340B Report webinar Oct. 28 about 340B’s future after the November midterm elections.
The event from 1:00 to 2:15 p.m. Eastern is an exclusive free
…Changing the 340B patient definition “might reduce hospitals’ and physicians’ incentives to consolidate,” but to what extent “remains highly uncertain,” the Congressional Budget Office says in a new policy analysis.
House Budget Committee Chair John Yarmuth (D-Ky.) asked CBO to
…Drug manufacturer UCB has sued in federal court to overturn federal health officials’ finding that the Belgian company’s restrictions on 340B pricing when covered entities use contract pharmacies are illegal and must stop or the company could face civil monetary
…As we first reported Friday in a news alert, a federal appeals court in Philadelphia has tentatively scheduled oral arguments in three key 340B contract pharmacy lawsuits for Nov. 15.
The clerk of the U.S. Third Circuit Court of Appeals
…The U.S. Health Resources and Services Administration (HRSA) has granted Florida and South Carolina 340B program enrollment flexibility, as public health emergencies (PHEs) were declared in both states in the aftermath of Hurricane Ian, which left widespread destruction, flooding, and
…