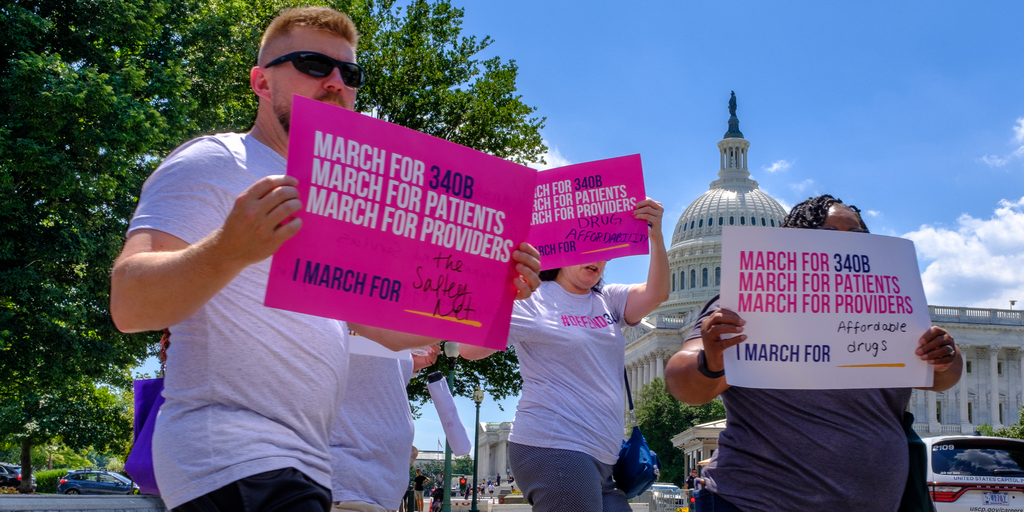The U.S. Senate on Sunday rejected a Republican amendment to Democratic drug pricing and climate change legislation that would have required federally qualified health centers to make insulin and injectable epinephrine available to patients at or below 340B price.
But
…