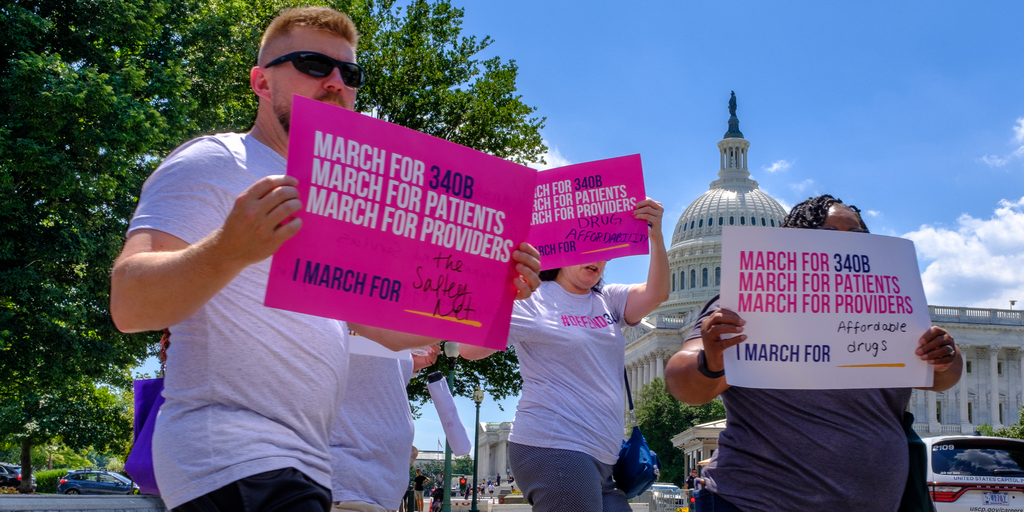The U.S. Senate on Sunday passed historic legislation to let Medicare negotiate the prices of a small number of costly medicines and require drug manufacturers to pay Medicare rebates when prices paid by Medicare rise faster than inflation.
Language in
…