Drug manufacturers Novartis and United Therapeutics (UT) last night urged a federal appeals court in Washington, D.C., to affirm a lower court’s November 2021 judgement that the 340B statute does not stop manufacturers from attaching any conditions to 340B drug
…Category: Federal
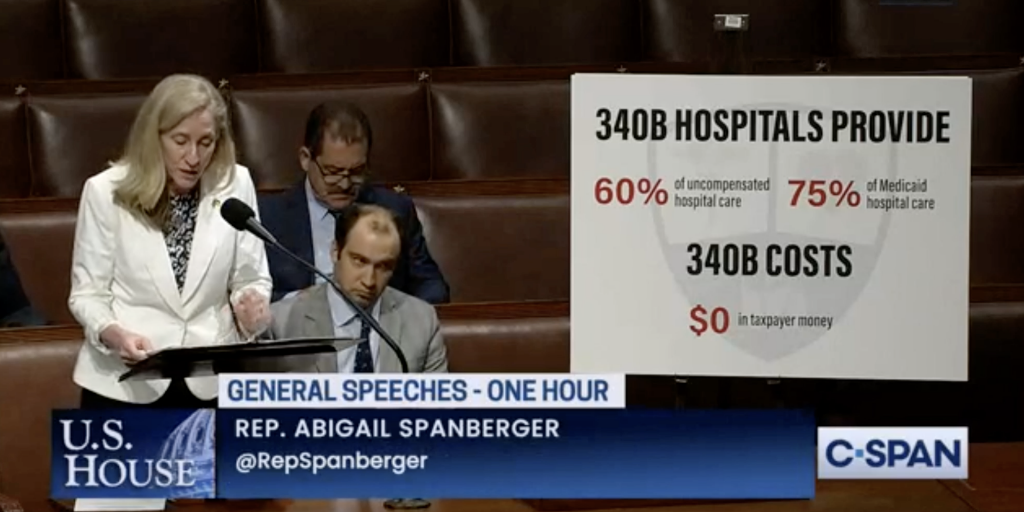
Seven U.S. representatives—five Democrats and two Republicans—lauded the 340B program on the House floor Tuesday night during an hour of speeches organized by Rep. Abigail Spanberger (D-Va.) and co-hosted by Rep. John Rose (R-Tenn.).
The event was postponed twice before
…Just months after deadlocking, the U.S. Federal Trade Commission voted unanimously on Tuesday to launch an investigation into pharmacy benefit managers’ business practices to assess their impact on prescription drug access and affordability.
Groups that represent 340B health care providers
Drug manufacturers AstraZeneca and Sanofi have asked a 340B administrative dispute resolution panel to dismiss the National Association of Community Health Centers’ claims that its members are being overcharged due to the companies’ denied or restricted 340B sales when health
…Generic drug maker e5 Pharma is giving 340B covered entities refunds for overcharges on six NDCs of the barbiturate phenobarbital during Q1 2021, according to a public notice on the U.S. Health Resources and Services Administration (HRSA) website.
Florida-based e5
…A federal district court’s ruling last October that federal law requires drug manufacturers to deliver 340B purchased drugs to contract pharmacies conflicts with two other courts’ conclusions, “upsets the delicate statutory balance Congress struck, and raises serious constitutional problems,” drug
…Pharmacy benefit managers’ discriminatory business practices against 340B hospitals boost PBMs’ financial bottom lines while hurting the hospitals’ mission to serve vulnerable patients, groups that represent or work with 340B hospitals and their pharmacists recently told the Federal Trade Commission
…Safety net healthcare providers increasingly have turned to the states to address challenges to the 340B program, 340B Report Publisher and CEO Ted Slafsky says in his latest column for medication management solutions company Omnicell.
“In just four years, an
…Overreliance on 340B drug discount revenues to fund HIV-prevention services incentivizes clinics to prescribe high-cost medicines instead of cheaper effective options, “with dire consequences for [preexposure prophylaxis] PrEP access, impact, and equity,” says an opinion article published last weekend in
…