Congress needs to give the U.S. Health Resources and Services Administration (HRSA) comprehensive regulatory authority over the 340B program to resolve the dispute over the 340B contract pharmacy program, the Biden administration said yesterday in its proposed budget—although it did
…Category: Federal
Arkansas health centers asked a federal court yesterday to let them intervene in the drug industry lawsuit challenging the state’s one-of-a-kind law protecting 340B contract pharmacy arrangements.
Community Health Centers of Arkansas (CHCA) filed the motion yesterday along with
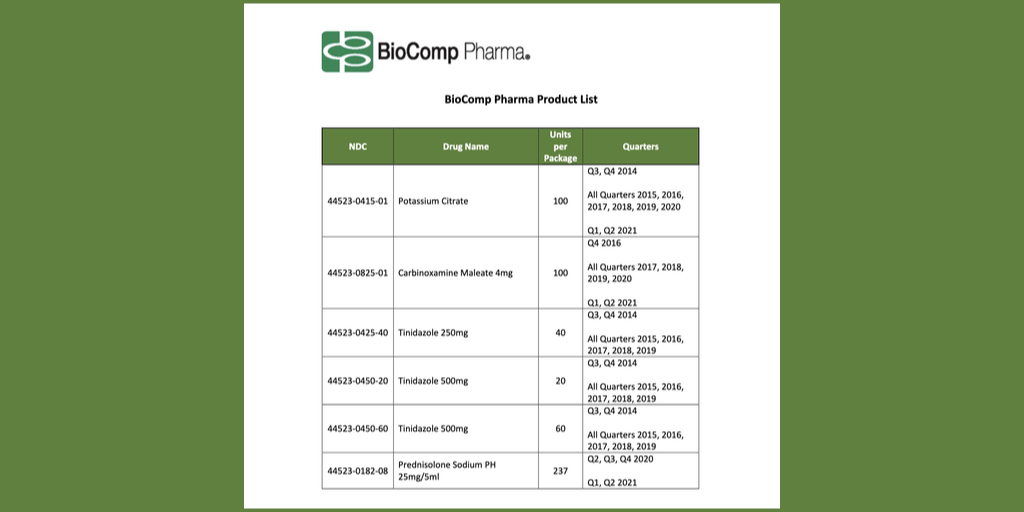
…Generic-drug manufacturer BioComp is offering covered entities refunds for lower 340B prices that it did not pay on six NDCs for periods over eight years, starting in Q3 2013 and ending in Q2 2021.
BioComp, a wholly-owned subsidiary of San
…The federal government has decided to intervene in a whistleblower lawsuit that accuses a Tennessee health system of paying Memphis-area cancer physicians for referrals that allegedly generated about $70 million a year in profits for the health system from billings
…California health centers told a federal judge last week that the state’s arguments for dismissing the centers’ lawsuit to reverse a new state Medicaid policy that deprives them of millions of dollars in 340B drug reimbursement lack merit.
Community Health
…News Alert
HRSA Starts Contacting Hospitals Forced Out of 340B During Pandemic About Possible Reinstatement
UPDATE Friday, March 18, 2022, 1:30 p.m. EDT—The U.S. Health Resources and Services Administration (HRSA) told 340B Report shortly after this story was published, “To be eligible, [a] hospital’s termination must have been as a result of actions taken by
…Medicare likely would pay 340B hospitals 106% of the average sales price (ASP) for Aduhelm—Biogen’s high-cost biologic drug for early-stage Alzheimer’s disease—for the first two or three years it is on the market, not the ASP minus 22.5% 340B hospital
…The U.S. Centers for Medicare & Medicaid Services (CMS) asked a federal district judge in Sacramento last week to dismiss California health centers’ claims for relief over CMS’s approval of the state’s transfer of Medicaid managed care drug benefits to
…A federal district judge on Friday entered his final orders and judgement in AstraZeneca’s 340B contract pharmacy lawsuit, clearing the way for the government to file its anticipated appeal.
Judge Leonard Stark of the District of Delaware closed the first
…President Biden is scheduled this afternoon to sign a $1.5 trillion federal appropriations bill with language to help some hospitals forced out of the 340B program during the COVID-19 pandemic.
The bill also gives the U.S. Health Resources and Services
…